Abstract
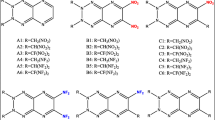
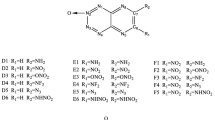
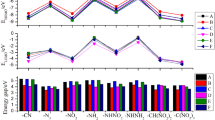
Density functional theory (DFT) was used to study the molecular geometries, electronic structures, heats of formation in gas phase and in condensed phase, energetic properties, and thermal stabilities of triazole derivatives. The results show that the properties are associated with the different substituents and substitution positions in the parent ring. The symmetric structures and hyperconjugation systems both contribute to the thermal stabilities of the triazole derivatives. It is found that the group –N3 is an effective structural unit for improving the gas phase heat of formation. The calculated detonation properties indicate that –NO2, –ONO2, –N3, –NF2, and –CH(NO2)2 groups are very useful for enhancing the detonation velocities and detonation pressures. Thirteen compounds have better detonation properties than that of HMX (1,3,5,7-tetranitro-1,3,5,7-tetrazocane). According to the quantitative data of energy and thermal stability for a nitrogen-rich high energetic compound, 20 out of 56 studied compounds may be considered as potential candidates with enhanced performance and reduced sensitivity.

Density functional theory was used to study the energetic properties and thermal stabilities of the triazole derivatives. Thirteen compounds have better detonation properties than those of HMX (1,3,5,7-tetranitro-1,3,5,7-tetrazocane). Twenty compounds may be considered as potential candidates with enhanced performance and reduced sensitivity.




Similar content being viewed by others
References
Klapotke T M and Witkowski T G 2015 Propell. Explos. Pyrot. 40 366
Fischer D, Klapotke T M and Stierstorfer J 2015 Angew. Chem. Int. Ed. 54 10299
Yedukondalu N and Vaitheeswaran G 2015 J. Chem. Phys. 143 064508
Wei H, He C L, Zhang J H and Shreeve J M 2015 Angew. Chem. Int. Ed. 54 9367
Piercey D G, Chavez D E, Heimsch S, Kirst C, Klapotke T M and Stierstorfer J 2015 Propell. Explos. Pyrot. 40 491
Wu J T, Zhang J G, Yin X, Cheng Z Y and Xu C X 2015 New J. Chem. 39 5265
Klapotke T M, Schmid P C, Schnell S and Stierstorfer J 2015 J. Mater. Chem. A 3 2658
Guo Y X, Feng X, Han T Y, Wang S, Lin Z G, Dong Y P and Wang B 2014 J. Am. Chem. Soc. 136 15485
Becuwe A and Delclos A 1989 Proceedings of the Ninth International Symposium on Detonation (Arlington, VA: Office of the Chief of Naval Research) pp. 1008–1013
Singh G, Kapoor I P S, Tiwari S K and Felix P S 2001 J. Hazard. Mater. 81 67
Lee J S and Jaw K S 2006 J. Therm. Anal. Calorim. 85 463
Dippold A A, Klapotke T M, Martin F A and Wiedbrauk S 2012 Eur. J. Inorg. Chem. 2429
Watenberg C, Charrne P and Laval F 1995 Propell. Explos. Pyrot. 20 23
Tang Y X, Gao H X, Parrish D A and Shreeve J M 2015 Chem. Eur. J. 21 11401
Wu Q, Zhu W H and Xiao H M 2014 J. Mol. Model. 20 2441
Tang Y X, Yang H W, Wu B, Ju X H, Lv C X and Cheng G B 2013 Angew. Chem. Int. Ed. 52 1
Klapotke T M, Schmid P C, Schnell S and Stierstorfer J 2015 Chem. Eur. J. 21 9219
Bian C M, Wang K, Liang L X, Zhang M, Li C and Zhou Z M 2014 Eur. J. Inorg. Chem. 6022
Srinivas D, Ghule V D and Muralidharan K 2014 RSC Adv. 4 7041
He P, Zhang J G, Wang K, Yin X, Jin X and Zhang T L 2015 Phys. Chem. Chem. Phys. 17 5840
Li X H and Zhang R Z 2014 J. Chem. Sci. 126 1753
Zhao G Z and Lu M 2013 J. Mol. Model. 19 3403
Singh R, Singh H J and Sengupta S K 2015 J. Chem. Sci. 127 1099
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas O, Foresman J B, Ortiz J V, Cioslowski J and Fox D J 2010 Gaussian 09, Revision C. 01 (Wallingford, CT: Gaussian Inc.)
Politzer P, Martinez J, Murray J S, Concha M C and Toro-Labbé A 2009 Mol. Phys. 107 2095
Becke A D 1992 J. Chem. Phys. 97 9173
Lee C, Yang W and Parr R G 1988 Phys. Rev. B 37 785
Zhao G Z and Lu M 2013 Struct. Chem. 24 139
Vo T T, Zhang J H, Parrish D A, Twamley B and Shreeve J M 2013 J. Am. Chem. Soc. 135 11787
Atkins P W 1982 In Physical Chemistry 2nd ed. (Oxford: Oxford University Press)
Rice B M, Pai S V and Hare J 1999 Combust. Flame 118 445
Politzer P, Lane P and Murray J S 2011 Cent. Eur. J. Energetic Mater. 8 39
Politzer P, Ma Y, Lane P and Concha M C 2005 Int. J. Quantum Chem. 105 341
Kamlet M J and Jacobs S J 1968 J. Chem. Phys. 48 23
Zhang X H and Yun Z H 1989 In Explosive Chemistry (Beijing: National Defence Industry Press)
Liu H, Wang F, Wang G X and Gong X D 2012 J. Comput. Chem. 33 1790
Li J S 2010 J. Phys. Chem. B 114 2198
Cao X Z, Song T Y and Wang X Q 1987 In Inorganic Chemistry (Beijing: Higher Education Press)
Scott A P and Radom L 1996 J. Phys. Chem. 100 16502
Dean J A 1999 In Lange’s Handbook of Chemistry 15th ed. (New York: McGraw-Hill)
David R L 2003–2004 In Handbook of Chemistry and Physics 84th ed. (Boca Raton: CRC Press)
Afeefy H Y, Liebman J F and Stein S E 2000 NIST Chemistry WebBook: NIST Standard Reference Database Number 69 (Gaithersburg: National Institute of Standards and Technology)
Yong P, Zhu W H and Xiao H M 2012 J. Mol. Model. 18 3125
Wei T, Zhang J J, Zhu W H, Zhang X W and Xiao H M 2010 J. Struct. Chem.: (THEOCHEM) 956 55
Huynh M H V, Hiskey M A, Chavez D E, Naud D L and Gilardi R D 2005 J. Am. Chem. Soc. 127 12537
Chavez D E, Hiskey M A and Gilardi R D 2000 Angew. Chem. Int. Ed. 39 1791
Talawar M B, Sivabalan R, Mukundan T, Muthurajan H, Sikder A K, Gandhe B R and Subhananda R 2009 J. Hazard. Mater. 161 589
Chung G, Schmidt M W and Gordon M S 2000 J. Phys. Chem. A 104 5647
Xiao H M, Xu X J and Qiu L 2008 In Theoretical Design of High Energy Density Materials (Beijing: Science Press)
Acknowledgments
This work was supported by the Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi and Natural Science Foundation of Shanxi Normal University (Grant No. ZR1504).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ZHAO, G., JIA, J. & WU, H. Design and selection of triazole-based compounds with high energetic properties and stabilities. J Chem Sci 128, 1223–1236 (2016). https://doi.org/10.1007/s12039-016-1117-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1117-x