Abstract
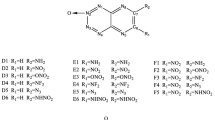
Based on the full optimized molecular geometric structures at B3LYP/6-311++G**level, the densities (ρ), heats of formation (HOFs), detonation velocities (D) and pressures (P) for a series of ditetrazoles derivatives, were investigated to look for high energy density materials (HEDMs). The results show that the influence of different substituted groups on HOFs has the order of -N3>-CN>-NH2>-NO2>-NF2>-ONO2>-H>-CH3>-CF3. The introduction of -CF3 groups is more favourable for increasing the density and the introduction of -CH3 groups is not favourable for increasing the density. In addition, all the series combined with -NF2 group except B-NF2 all have higher densities, larger D and P. F-NF2 may be regarded as the potential candidates of HEDMs because of the largest detonation velocity and pressure among these derivatives. The energy gaps between the HOMO and LUMO of the studied compounds are also investigated.

Computational results show that ditetrazoles combined with -NF2 group except B-NF2 have higher densities, larger D and P. F-NF2 may be regarded as the potential candidate of HEDMs because of the largest detonation velocity and pressure among these derivatives.





Similar content being viewed by others
References
Huynh M H V, Hiskey M A, Pollard C J, Montoya D P, Hartline E L and Gilardi R D 2004 J. Energ. Mater. 22 217
Huynh M H V, Hiskey M A, Chavez D E, Naud D L and Gilardi R D 2005 J. Am. Chem. Soc. 127 12537
Gutowski K E, Rogers R D and Dixon D A 2007 J. Phys. Chem. B. 111 4788
Talawar M B, Sivabalan R, Senthilkumar N, Prabhu G and Asthana S N 2004 J. Hazard. Mater. 113 11
Chavez D E, Hiskey M A and Gilardi R D 2000 Angew. Chem. Int. Ed. 39 1791
Kerth J and Lobbecke S 2002 Propellants Explos. Pyrotech. 27 111
Neutz J, Grosshardt O, Schaufele S, Schuppler H and Schweikert W 2003 Propellants Explos. Pyrotech. 28 181
Huynh M H V, Hiskey M A, Hartline E L, Montoya D P and Gilardi R D 2004 Angew. Chem. Int. Ed. 43 4924
Churakov A M, Smirnov O Y, Ioffe S L, Strelenko Y A and Tartakovsky V A 2002 Eur. J. Org. Chem. 14 2342
Joo Y H, Gao H X, Zhang Y Q and Shreeve J M 2010 Inorg. Chem. 49 3282
Guo Y, Tao G H, Zeng Z, Gao H X, Parrish D A and Shreeve J M 2010 Chem. Eur. J. 16 3753
Klapötke T M and Stierstorfer J 2009 J. Am. Chem. Soc. 131 1122
Joo Y B and Shreeve J M 2009 Angew. Chem. Int. Ed. 48 564
Li X H, Zhang R Z and Zhang X Z 2013 Struct. Chem. 24 393
Li X H, Zhang R Z and Zhang X Z 2011 Struct. Chem. 224 577
Zhang R Z, Li X H and Zhang X Z 2012 J. Chem. Sci. 124 995
Alexander D, Thomas Klapke M and Franz Martin A 2011 Z. Anorg. Allg. Chem. 637 1181
Ravi P, Girish Gore M, Surya Tewari P and Arun Sikder K 2012 Propellants Explos. Pyrotech. 37 52
Zhang C, Zhu W and Xiao H 2011 Comput. Theor. Chem. 967 257
Joo Y H and Shreeve J M 2009 Angew. Chem. Int. Ed. 48 564
Wei T, Wu J, Zhu W, Zhang C and Xiao H 2012 J. Mol. Model. 18 3467
Zhang X, Zhu W and Xiao H 2010 J. Phys. Chem. A. 114 603
Becke D 1992 J. Chem. Phys. 97 9173
Hariharan P C and Pople J A 1973 Theor. Chim. Acta. 28 213
Li X H, Zhang R Z and Zhang X Z 2010 J. Hazard. Mater. 183 622
Li X H, Cheng Q D and Zhang X Z 2010 J. Energ. Mater. 28 251
Xu X J, Xiao H M, Ju X H, Gong X D and Zhu W H 2006 J. Phys. Chem. A. 110 5929
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Zakrzewski V G, Montgomery J A, Stratmann R E, Burant J C, Dapprich S, Millam J. M, Daniels A. D, Kudin K N, Strain M. C, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson G A, Ayala P Y, Cui Q, Morokuma K, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Cioslowski J, Ortiz J V, Baboul A G, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R L, Fox D J, Keith T, Al- Laham M A, Peng C Y, Nanayakkara A, Gonzalez C, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Andres J L, Gonzalez C, Head Gordon M, Replogle E S and Pople J A 2003 GAUSSIAN 03, Revision B.02, Gaussian Inc.: Pittsburgh PA
Hehre W J, Radom L and Schleyer P V R 1986 In Ab Initio molecular orbital theory (New York: Wiley) p 124
Rice B M, Sahu S and Owens F J 2002 J. Mol. Struct. Theochem 583 69
Kamlet M J and Jacobs S J 1968 J. Chem. Phys. 48 23
Zhang X H and Yun Z H 1989 In Explosive Chemistry (Beijing: National Defense Industry Press) p 198
Politzer P, Martinez J, Jane Murray S, Monica Concha C and Alejandro T 2009 Mol. Phys. 107 2095
Lide DR 2004 In Handbook of chemistry and physics, 84th edn. (Boca Raton: CRC Press LLC) p 54
NIST Standard Reference Data Base Number 69, (http://webbook.nist.gov/chemistry)
Balepin A A, Lebedev V P, Miroshnichenko E A, Koldobskii G I, Ostovskii V A, Larionov B P, Gidaspov B V and Lebedev Yu A 1977 Svoistva Veshchestv Str Mol. 2 93
Li X H, Tang Z X, Zhang X Z and Yang X D 2009 J. Hazard. Mater. 165 372
Li X H, Yin G X and Zhang X Z 2012 Chinese J. Chem. Phys. 25 545
Li X H, Fu Z M and Zhang X Z 2012 Struct Chem. 23 515
Li X H, Zhang R Z and Zhang X Z 2010 J. Hazard. Mater. 183 622
Talawar M B, Sivabalan R, Mukundan T, Muthurajan H, Sikder A K, Gandhe B R and Rao A S 2009 J. Hazard. Mater. 161 589
Gilardi R, Flippen-Anderson J L and Evans R 2002 Acta Crystallogr. E58 o972
Fleming I 1976 Frontier orbitals and organic chemical reactions (New York: John Wiley and Sons) p 157
Acknowledgements
We thank the National Natural Science Foundation of China (Grant U1304111), China Postdoctoral Science Foundation (No. 2013M531361) and Jiangsu Planned Projects for Postdoctoral Research Funds (No. 1201015B) for their support to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
XIAO-HONG, L., RUI-ZHOU, Z. Computational studies on energetic properties of nitrogen-rich energetic materials with ditetrazoles. J Chem Sci 126, 1753–1762 (2014). https://doi.org/10.1007/s12039-014-0665-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0665-1