Abstract
Equimolar reaction of copper(I) bromide with 2-thiouracil (tucH2) in acetonitrile-methanol formed a light yellow solid which on subsequent treatment with a mole of triphenyl phosphine (PPh3) in chloroform has yielded a sulfur-bridged dinuclear complex, [Cu2Br2(μ-S-tucH2)2(PPh3)2] ⋅2CHCl3 1. A reaction of copper(I) bromide with two moles of 2,4-dithiouracil (dtucH2) in acetonitrile-methanol followed by addition of two moles of PPh3, designed to form [Cu(μ-S,S-dtuc)2(PPh3)4Cu] 2a, instead resulted in the formation of previously reported polymer, {CuBr(μ-S,S-dtucH2)(PPh3)}n 2. Reaction of copper(I) iodide with 2-thiouracil (tucH2) and PPh3 in 1:1:2 molar ratio (Cu:H2tuc:PPh3) as well as that of copper(I) thiocyanate with pyridine-2-thione (pySH) or pyrimidine-2-thione (pymSH) and PPh3 in similar ratio, yielded an iodo-bridged unsymmetrical dimer, [(PPh3)2(μ-I)2Cu(PPh3)] 3 and thiocyanate bridged symmetrical dimer, [(PPh3)2Cu(μ-N,S- SCN)2Cu(PPh3)2] 4, respectively. In both the latter reactions, thio-ligands which initially bind to Cu metal center, are de-ligated by PPh3 ligand. Crystal data: 1, P21/c: 173(2) K, monoclinic, a, 13.4900(6); b, 17.1639(5); c, 12.1860(5) Å; β, 111.807(5) ∘; R, 5.10%; 2, Pbca: 296(2) K, orthorhombic, a, 10.859(3); b, 17.718(4); c, 23.713(6) Å; α=β=γ, 90 ∘; R, 4.60%; 3, P21: 173(2) K, monoclinic, a, 10.4208(7); b, 20.6402(12); c, 11.7260(7) Å; β, 105.601(7)∘; R, 3.97%; 4, P-1: 173(2) K, triclinic, a, 10.2035(4); b, 13.0192(5); c, 13.3586(6) Å; α, 114.856(4); β, 92.872(4)∘; γ, 100.720(4)∘; R, 3.71%. ESI-mass studies reveal different fragments of complexes.

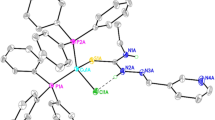
Sulfur-bridged dimer [Cu2Br2(μ-S-tucH2)2(PPh3)2]·2CHCl3 1 is the first example of a dinuclear complex of 2-thiouracil/ 2,4-dithiouracil class of ligands.











Similar content being viewed by others
References
Raper E S 1996 Coord. Chem. Rev. 153 199
Raper E S 1994 Coord. Chem. Rev. 129 91
Raper E S 1997 Coord. Chem. Rev. 165 475
Garcia-Vazquez J A, Romero J and Sousa A 1999 Coord. Chem. Rev. 193 691
Akrivos P D 2001 Coord. Chem. Rev. 213 181
Aslanidis P, Cox P J and Tsipis A C 2010 Dalton Trans. 39 10238
Kimani M M, Bayse C A and Brumaghim J L 2011 Dalton Trans. 40 3711
Karagiannidis P, Aslanidis P, Kessissoglou D P, Krebs B and Dartmann M 1989 Inorg. Chim. Acta 156 47
Leconite C, Skoulika St, Aslanidis P, Karagiannidis P and Papastefanou St 1989 Polyhedron 8 1103
Lobana T S, Kaur P, Castineiras A, Turner P and Failes T W 2008 Struct. Chem. 19 727
Lobana T S, Sharma R, Sharma R, Mehra S, Castineiras A and Turner P 2005 Inorg. Chem. 44 1914
Lobana T S, Sultana R, Hundal G and Butcher R J 2010 Dalton Trans. 39 7870
Ozturk I, Filimonova S, Hadjikakou S K, Kourkoumelis N V, Dokorou M. M J, Tasiopoulos A J, Barsan M M, Butler I S, Milaeva E R, Balzarini J and Hadjiliadis N 2010 Inorg. Chem. 49 488
Kela U and Vijayavargiya R 1981 Biochem. J. 193 799
Dubler E and Gyr E 1988 Inorg. Chem. 27 1466
Masoud M S, Soayed A A and El-Husseiny A F 2012 Spectrochim. Acta Part A 99 365
Hunt G W, Griffith E A H and Amma E L 1976 Inorg. Chem. 15 2993
Lobana T S, Sandhu A K, Sultana R, Butcher R J, Castineiras A and Jasinski J P 2014 RSC Adv. 4 30511
Yamanari K, Nozaki T, Fuyuhiro A, Kushi Y and Kaizaki S 1996 J. Chem. Soc., Dalton Trans. 2851
Yamanari K, Okusako K, Kushi Y and Kaizaki S 1992 J. Chem. Soc., Dalton Trans. 1621
Yamanari K, Kida M, Yamamoto M, Fujihara T, Fuyuhiro A and Kaizaki S 1995 J. Chem. Soc., Dalton Trans. 2627
Yamanari K, Kida M, Yamamoto M, Fujihara T, Fuyuhiro A and Kaizaki S 1993 Chem. Lett. 1865
Hoskins B F, Zhenrong L and Tiekink E R T 1989 Inorg. Chim. Acta 158 7
Harker C S W, Tiekink E R T and Whitehouse M W 1991 Inorg. Chim. Acta 181 23
Cookson P D and Tiekink E R T 1993 J. Cryst. Spectr. Res. 23 231
Cookson P D and Tiekink E R T 1994 Z. Kristallogr. 209 749
Cookson P D and Tiekink E R T 1994 J. Chem. Cryst. 24 805
Romero M A, Sanchez M P, Quiros M, Sanchez F, Salas J M and Moreno M N 1993 Can. J. Chem. 71 29
Ruf M, Weis K and Vahrenkamp H 1997 J. Am. Chem. Soc. 36 2130
Garcia-Tasende M S, Suarez-Gimeno M I, Sanchez A, Casas J S, Sordo J and Castellano E E 1990 J. Organomet. Chem. 384 19
Garcia-Tasende M S, Suarez-Gimeno M I, Sanchez A, Casas J S, Sordo J, Castellano E E and Mascarenhas Y P 1987 Inorg. Chem. 26 3818
Aslanidis P, Cox P J, Kaltzoglou A and Tsipis A C 2006 Eur. J. Inorg. Chem. 334
Sultana R, Lobana T S, Sharma R, Castineiras A, Akitsu T, Yahagi K and Aritake Y 2010 Inorg. Chim. Acta 363 3432
Lobana T S, Sultana R, Butcher R J, Jasinski J P, Golen J A, Castineiras A, Pröpper K, Fernandez F J and Vega 2013 J. Organomet. Chem. 745–746 460
Yamanari K, Kushi Y, Fuyuhiro A and Kaizaki S 1993 J. Chem. Soc., Dalton Trans. 403
Corbin D R, Francesconi L C, Hendrickson D N and Stucky G 1979 J. Chem. Soc., Chem. Commun. 248
Landgrafe C and Sheldrick W S 1986 J. Chem. Soc., Dalton Trans. 989
Raper E S, Creighton J R, Wilson J D, Clegg W and Milne A 1989 Inorg. Chim. Acta 155 85
Bowmaker G A, Hanna J V, Skelton B W and White A H 2009 Chem. Commun. 2168
Lemos S S, Camargo M A, Cardoso Z Z, Deflon V M, Forsterling F H and Hagenbach A 2001 Polyhedron 20 849
Popovic Z, Pavlovic G, Calogovic D M, Soldin Z, Rajic M, Topic D V and Kovacek D 2000 Inorg. Chim. Acta 306 142
Lobana T S, Sharma R, Hundal G and Butcher R J 2006 Inorg. Chem. 45 9402
Oxford Diffraction 2009 CrysAlis Pro CCD and CrysAlis Pro RED (Yarnton, England: Oxford Diffraction Ltd.)
Sheldrick G M 2008 Acta Crystallogr. Sect. A 64 112
Altomare A, Cascarano G, Giacovazzo C and Guagliardi A 1993 J. Appl. Crystallogr. 26 343
Wilson A J C 1995 In International Tables for Crystallography Vol. C (Netherlands: Kluwer Academic)
Lobana T S and Castineriras A 2002 Polyhedron 21 1603
Huheey J E and Keiter R L 1993 In Inorganic Chemistry; Principal of Structure and Reactivity 4 th edn. (New York: Harper Collins)
Pauling L 1960 In The Nature of the Chemical Bond 3rd edn. (New York: Cornell University Press)
Karagiannidis P, Aslanidis P, Kessissoglou D P, Krebs B and Dartmann M 1989 Inorg. Chim. Acta 156 47
Aslanidis P, Hadjikakou S K, Karagiannidis P, Kojic-Prodic B and Luic M 1994 Polyhedron 13 3119
Lobana T S, Paul S and Castineiras A 1997 Polyhedron 16 4023
Karagiannidis S K, Hadjikakou S K, Aslanidis P and Hountas A 1990 Inorg. Chim. Acta 178 27
Eller P G, Kubas G J and Ryan R W 1977 Inorg. Chem. 16 2454
Homsy N K, Noltemeyer M, Roesky H W, Schmidt H-G and Sheldrick G M 1984 Inorg. Chim. Acta 90 L59
Acknowledgements
Financial assistance in the form of Emeritus Scientist Grant [21(0904)/12-EMR-II] to T.S. Lobana, from the Council of Scientific and Industrial Research (CSIR), New Delhi, and grant from the Department of Science and Technology (DST) for X-ray diffractometer to Department of Chemistry, GNDU, Amritsar are gratefully acknowledged. JPJ acknowledges the NSF-MRI program (Grant No. CHE-1039027) for funds to purchase the X-ray diffractometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB21EZ, UK. Copies of the data can be obtained free of charge on quoting the depository numbers CCDC 1405753 for 1, 1405754 for 2, 1405755 for 3 and 1405756 for 4 (Fax: +44-1223-336-033; E-Mail: deposit@ccdc.cam.ac.uk, http://www.ccdc.cam.ac.uk). ESI mass data area in Supplementary Information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
LOBANA, T.S., KAUR, A., SHARMA, R. et al. Synthesis, molecular structures and ESI-mass studies of copper(I) complexes with ligands incorporating N, S and P donor atoms. J Chem Sci 127, 1859–1869 (2015). https://doi.org/10.1007/s12039-015-0953-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0953-4