Abstract
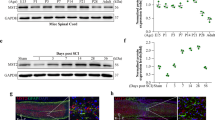
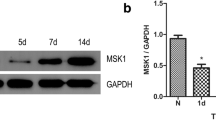
Spinal cord injury is a severe clinical problem, and research searching activity molecular that can promote spinal cord injury repairing is very prevalent. Mst3b can promote repair of damaged peripheral nerves and the optic nerve, but has been rarely reported in spinal cord injury research. Through detecting its expression in different periods of injured spinal cord, we found that the expression of Mst3b was significantly upregulated in injured spinal cord neurons. Increasing Mst3b expression using adenovirus in vivo and in vitro promoted axonal regeneration of spinal cord neurons, which led to behavioral and electrophysiological improvement. Downregulation of Mst3b level had the adverse effects. Increasing Mst3b expression in PC12 cells resulted in an elevation of P42/44MAPK and LIMK/Cofilin activation. These results identified Mst3b as a powerful regulator for promoting spinal cord injury recovery through the P42/44MAPK and LIMK/Cofilin signaling pathways.








Similar content being viewed by others
References
Burns AS, O’Connell C (2012) The challenge of spinal cord injury care in the developing world. J Spinal Cord Med 35–38
Cripps RA, Lee BB, Wing P, Weerts E, Mackay J, Brown D (2011) A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord 49:493–501
Fawcett JW et al (2007) Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45:190–205
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294–301
Park KK et al (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963–966
Buchli AD, Schwab ME (2005) Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med 37:556–567
Woolf CJ (2003) No Nogo: now where to go? Neuron 38:153–156
Harel NY, Strittmatter SM (2006) Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci 7:603–616
Schwab ME (2002) Repairing the injured spinal cord. Science 295:1029–1031
Varma AK et al (2013) Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res 38:895–905
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627
Parikh P et al (2011) Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acad Sci U S A 108:E99–E107
Neumann S, Woolf CJ (1999) Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23:83–91
Leberer E, Dignard D, Harcus D, Thomas DY, Whiteway M (1992) The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J 11:4815–4824
Zhou TH et al (2000) Identification of a human brain-specific isoform of mammalian STE20-like kinase 3 that is regulated by cAMP-dependent protein kinase. J Biol Chem 275:2513–2519
Irwin N, Li YM, O’Toole JE, Benowitz LI (2006) Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci U S A 103:18320–18325
Zai L et al (2011) Inosine augments the effects of a Nogo receptor blocker and of environmental enrichment to restore skilled forelimb use after stroke. J Neurosci 31:5977–5988
Lorber B, Howe ML, Benowitz LI, Irwin N (2009) Mst3b, an Ste20-like kinase, regulates axon regeneration in mature CNS and PNS pathways. Nat Neurosci 12:1407–1414
Bernard O (2007) Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol 39:1071–1076
Dong Q, Ji YS, Cai C, Chen ZY (2012) LIM kinase 1 (LIMK1) interacts with tropomyosin-related kinase B (TrkB) and mediates brain-derived neurotrophic factor (BDNF)-induced axonal elongation. J Biol Chem 287:41720–41731
Yoshida Y et al (2013) Transection method for shortening the rat spine and spinal cord. Exp Ther Med 5:384–388
Taylor L, Jones L, Tuszynski MH, Blesch A (2006) Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci 26:9713–9721
Lu P et al (2012) Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150:1264–1273
Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI (2000) Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 20:4615–4626
Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME (2004) The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7:269–277
Wang Y, Zhang X, Zhang Y, Xu H, Fang G (2011) Expression and localization of IL-18 in the hypothalamic-pituitary-ovarian axis of non-pregnant, pregnant, and abortive rats. J Reprod Immunol 92:45–53
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139:244–256
Bradbury EJ et al (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640
Xu L et al (2012) Neural stem cells enhance nerve regeneration after sciatic nerve injury in rats. Mol Neurobiol 46:265–274
Jin Y, Fischer I, Tessler A, Houle JD (2002) Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol 177:265–275
Zhao RR et al (2011) Lentiviral vectors express chondroitinase ABC in cortical projections and promote sprouting of injured corticospinal axons. J Neurosci Methods 201:228–238
Jiang XY et al (2006) Methods for isolating highly-enriched embryonic spinal cord neurons: a comparison between enzymatic and mechanical dissociations. J Neurosci Methods 158:13–18
Rosso S et al (2004) LIMK1 regulates Golgi dynamics, traffic of Golgi-derived vesicles, and process extension in primary cultured neurons. Mol Biol Cell 15:3433–3449
Schaden H, Stuermer CA, Bahr M (1994) GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. J Neurobiol 25:1570–1578
Lowery LA, Van Vactor D (2009) The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 10:332–343
Marsh L, Letourneau PC (1984) Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol 99:2041–2047
Flynn KC et al (2012) ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron 76:1091–1107
Gao Y et al (2004) Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44:609–621
David S, Lacroix S (2003) Molecular approaches to spinal cord repair. Annu Rev Neurosci 26:411–440
Wang Y et al (2012) BDNF and NT-3 expression by using glucocorticoid-induced bicistronic expression vector pGC-BDNF-IRES-NT3 protects apoptotic cells in a cellular injury model. Brain Res 1448:137–143
Dumont RJ et al (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24:254–264
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76:319–370
Ylera B et al (2009) Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol 19:930–936
David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214:931–933
Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J (2006) Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci 26:7405–7415
Richardson PM, McGuinness UM, Aguayo AJ (1980) Axons from CNS neurons regenerate into PNS grafts. Nature 284:264–265
Kadoya K et al (2009) Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron 64:165–172
Ling P, Lu TJ, Yuan CJ, Lai MD (2008) Biosignaling of mammalian Ste20-related kinases. Cell Signal 20:1237–1247
Cowley S, Paterson H, Kemp P, Marshall CJ (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3 T3 cells. Cell 77:841–852
Markus A, Zhong J, Snider WD (2002) Raf and akt mediate distinct aspects of sensory axon growth. Neuron 35:65–76
Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD (2007) Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci 10:598–607
Kaplan DR, Miller FD (2000) Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10:381–391
Hur Saijilafu EM, Zhou FQ (2012) Growing the growth cone: remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci 35:164–174
Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC (2006) TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A 103:10444–10449
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81171142 to F.H. and No. 81001591 to H.H.), Science and Technology Development Planning Project of Shandong Province (No. 2013G0021816 to F.H.), and the Natural Science Foundation of Shandong (No. ZR2010HQ065 to H.H.). We thank Fengchan Han for technical assistance, Xiuli Zhang for the supporting cell line, and the Electron Microscope Center for the transmission electron microscope analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yuqiang Zhang and Huaiqiang Hu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, Y., Hu, H., Tian, T. et al. Mst3b Promotes Spinal Cord Neuronal Regeneration by Promoting Growth Cone Branching Out in Spinal Cord Injury Rats. Mol Neurobiol 51, 1144–1157 (2015). https://doi.org/10.1007/s12035-014-8785-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8785-7