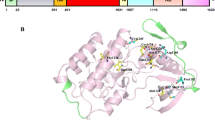
Abstract
The non-receptor protein tyrosine phosphatase is a class of enzymes that catalyze the dephosphorylation of phosphotyrosines in protein molecules. They are involved in cellular signaling by regulating the phosphorylation status of a variety of receptors and signaling molecules within the cell, thereby influencing cellular physiological and pathological processes. In this article, we detail multiple non-receptor tyrosine phosphatase and non-receptor tyrosine phosphatase genes involved in the pathological process of brain disease. These include PTPN6, PTPN11, and PTPN13, which are involved in glioma signaling; PTPN1, PTPN5, and PTPN13, which are involved in the pathogenesis of Alzheimer’s disease Tau protein lesions, PTPN23, which may be involved in the pathogenesis of Epilepsy and PTPN1, which is involved in the pathogenesis of Parkinson’s disease. The role of mitochondrial tyrosine phosphatase in brain diseases was also discussed. Non-receptor tyrosine phosphatases have great potential for targeted therapies in brain diseases and are highly promising research areas.



Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMPR :
-
α-amino-3-hydroxy-5-methyl-4-isoxazolidinepropanoic acid receptor
- AD :
-
Alzheimer's disease
- Aβ :
-
Amyloid-beta
- CNS :
-
Central nervous system
- CTE :
-
Chronic traumatic encephalopathy
- EGFR :
-
Epidermal growth factor receptor
- EGFRvIII :
-
Epidermal growth factor receptor variant III
- FAS :
-
Fas-ligand
- FXS :
-
Fragile X syndrome
- GCS :
-
Glasgow coma scale
- GBM :
-
Glioblastoma
- GSK-3β :
-
Glycogen synthase kinase-3beta
- HePTP :
-
Hematopoietic tyrosine phosphatase
- HD :
-
Huntington's disease
- HIF-1/2α :
-
Hypoxia-inducible factors 1 and 2alpha
- KIM :
-
Kinase interaction motif
- LGG :
-
Low-grade gliomas
- MAPK :
-
Mitogenactivated protein kinase
- NFTs :
-
Neurofibrillary tangles
- NMDA :
-
n-methyl- d -aspartate receptor
- NMDAR :
-
n-methyl-d-aspartate receptor
- N-SH2 :
-
N-terminal domain
- PHF :
-
Paired helical filament
- PD :
-
Parkinson's disease
- PI3K :
-
Phosphoinositide 3-kinase
- PPFIBP1 :
-
PPFIA binding protein 1
- pi-miR-124 :
-
primary-miR-124
- PSP :
-
Progressive supranuclear palsy
- PP2A :
-
Protein phosphatase 2A
- PTK :
-
Protein tyrosine kinase
- PTPL1 :
-
Protein tyrosine phosphatase L1
- PTPMT1 :
-
Protein Tyrosine Phosphatase localized to the Mitochondrion 1
- PTPN :
-
Protein tyrosine phosphatase non-receptor type
- PTPN1 :
-
Tyrosine-protein phosphatase non-receptor type 1
- PTPN6 :
-
Tyrosine-protein phosphatase non-receptor type 6
- PTPN11 :
-
Tyrosine-protein phosphatase non-receptor type 11
- PTPN13 :
-
Tyrosine-protein phosphatase non-receptor type 13
- PTPN23 :
-
Tyrosine-protein phosphatase non-receptor type 23
- PTPs :
-
Protein tyrosine phosphatase
- PTP1B :
-
Protein Tyrosine Phosphatase 1B
- RTK :
-
Receptor tyrosine kinase
- RISC :
-
RNA-induced silencing complex
- STAT3 :
-
Signal transducer and activator of transcription-3
- SHP1 :
-
Src homology domain-containing PTPs 1
- SHP2 :
-
Src homology domain-containing PTPs 2
- STEP :
-
Striatal enriched protein tyrosine phosphatase
- STEP46 :
-
Striatal enriched protein tyrosine phosphatase 46
- STEP61 :
-
Striatal enriched protein tyrosine phosphatase 61
- TBI :
-
Traumatic brain injury
References
Ostrom QT et al (2014) The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 16(7):896–913. https://doi.org/10.1093/neuonc/nou087
Alexander BM, Cloughesy TF (2017) Adult Glioblastoma. J Clin Oncol 35(21):2402–2409. https://doi.org/10.1200/JCO.2017.73.0119
Dai C, Holland EC (2001) Glioma models. Biochim Biophys Acta 1551(1):M19-27. https://doi.org/10.1016/s0304-419x(01)00027-0
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068. https://doi.org/10.1038/nature07385
Wang Q et al (2017) Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32(1):42-56.e6. https://doi.org/10.1016/j.ccell.2017.06.003
Brennan CW et al (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. https://doi.org/10.1016/j.cell.2013.09.034
Barzegar Behrooz A, Talaie Z, Jusheghani F, Łos MJ, Klonisch T, Ghavami S (2022) Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in Glioblastoma. Int J Mol Sci 23(3):1353. https://doi.org/10.3390/ijms23031353
Dong C et al (2021) PPFIBP1 induces glioma cell migration and invasion through FAK/Src/JNK signaling pathway. Cell Death Dis 12(9):827. https://doi.org/10.1038/s41419-021-04107-7
Ma C, Zhao G, Cruz MH, Siden A, Yakisich JS (2014) Translational gap in glioma research. Anticancer Agents Med Chem 14(8):1110–1120. https://doi.org/10.2174/1871520614666140825110907
Guishard AF, Yakisich JS, Azad N, Iyer AKV (2018) Translational gap in ongoing clinical trials for glioma. J Clin Neurosci 47:28–42. https://doi.org/10.1016/j.jocn.2017.10.001
Breijyeh Z, Karaman R (2020) Comprehensive review on Alzheimer’s Disease: causes and treatment. Molecules 25(24):5789. https://doi.org/10.3390/molecules25245789
Pavlovic D, Pekic S, Stojanovic M, Popovic V (2019) Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae’. Pituitary 22(3):270–282. https://doi.org/10.1007/s11102-019-00957-9
He R-J, Yu Z-H, Zhang R-Y, Zhang Z-Y (2014) Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin 35(10):1227–1246. https://doi.org/10.1038/aps.2014.80
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393(10172):689–701. https://doi.org/10.1016/S0140-6736(18)32596-0
Frontiers | Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes’ (2020) https://www.frontiersin.org/articles/10.3389/fneur.572976/full. Accessed 16 May 2023
Hunter T (1995) Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80(2):225–236. https://doi.org/10.1016/0092-8674(95)90405-0
Dempke WCM, Uciechowski P, Fenchel K, Chevassut T (2018) Targeting SHP-1, 2 and SHIP pathways: a novel strategy for cancer treatment? Oncology 95(5):257–269. https://doi.org/10.1159/000490106
Liu Q, Qu J, Zhao M, Xu Q, Sun Y (2020) Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res 152:104595. https://doi.org/10.1016/j.phrs.2019.104595
Freiss G, Chalbos D (2011) PTPN13/PTPL1: an important regulator of tumor aggressiveness. Anticancer Agents Med Chem 11(1):78–88. https://doi.org/10.2174/187152011794941262
Kostrzewa T, Styszko J, Gorska-Ponikowska M, Sledzinski T, Kuban-Jankowska A (2019) Inhibitors of Protein Tyrosine Phosphatase PTP1B with anticancer potential. Anticancer Res 39(7):3379–3384. https://doi.org/10.21873/anticanres.13481
Kumar A, Rana D, Rana R, Bhatia R (2020) Protein tyrosine phosphatase (PTP1B): a promising drug target against life-threatening ailments. Curr Mol Pharmacol 13(1):17–30. https://doi.org/10.2174/1874467212666190724150723
Chen P-J, Zhang Y-T (2022) Protein tyrosine phosphatase 1B (PTP1B): insights into its new implications in tumorigenesis. Curr Cancer Drug Targets 22(3):181–194. https://doi.org/10.2174/1568009622666220128113400
Eleftheriou P, Geronikaki A, Petrou A (2019) PTP1b inhibition, a promising approach for the treatment of diabetes type II. Curr Top Med Chem 19(4):246–263. https://doi.org/10.2174/1568026619666190201152153
Sharma B et al (2020) Recent advance on PTP1B inhibitors and their biomedical applications. Eur J Med Chem 199:112376. https://doi.org/10.1016/j.ejmech.2020.112376
Kamceva M, Benedict J, Nairn AC, Lombroso PJ (2016) Role of striatal-enriched tyrosine phosphatase in neuronal function. Neural Plast :8136925. https://doi.org/10.1155/2016/8136925
Karasawa T, Lombroso PJ (2014) Disruption of striatal-enriched protein tyrosine phosphatase (STEP) function in neuropsychiatric disorders. Neurosci Res 89:1–9. https://doi.org/10.1016/j.neures.2014.08.018
Xu J, Kurup P, Nairn AC, Lombroso PJ (2012) Striatal-enriched protein tyrosine phosphatase in Alzheimer’s disease. Adv Pharmacol 64:303–325. https://doi.org/10.1016/B978-0-12-394816-8.00009-X
Lombroso PJ, Murdoch G, Lerner M (1991) Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci U S A 88(16):7242–7246. https://doi.org/10.1073/pnas.88.16.7242
Venkitaramani DV et al (2009) Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse 63(1):69–81. https://doi.org/10.1002/syn.20608
Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14(3):311–317. https://doi.org/10.1016/j.conb.2004.04.001
Borders AS, de Almeida L, Van Eldik LJ, Watterson DM (2008) The p38alpha mitogen-activated protein kinase as a central nervous system drug discovery target. BMC Neurosci 9(Suppl 2):S12. https://doi.org/10.1186/1471-2202-9-S2-S12
Wu PH, Coultrap SJ, Browning MD, Proctor WR (2011) Functional adaptation of the N-methyl-D-aspartate receptor to inhibition by ethanol is modulated by striatal-enriched protein tyrosine phosphatase and p38 mitogen-activated protein kinase. Mol Pharmacol 80(3):529–537. https://doi.org/10.1124/mol.110.068643
Won S, Roche KW (2021) Regulation of glutamate receptors by striatal-enriched tyrosine phosphatase 61 (STEP61). J Physiol 599(2):443–451. https://doi.org/10.1113/JP278703
Snyder EM et al (2005) Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8(8):1051–1058. https://doi.org/10.1038/nn1503
Tautermann CS et al (2019) Allosteric activation of Striatal-Enriched protein tyrosine phosphatase (STEP, PTPN5) by a fragment-like molecule. J Med Chem 62(1):306–316. https://doi.org/10.1021/acs.jmedchem.8b00857
Nguyen T-H, Liu J, Lombroso PJ (2002) Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem 277(27):24274–24279. https://doi.org/10.1074/jbc.M111683200
Zhang Y et al (2008) The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci 28(42):10561–10566. https://doi.org/10.1523/JNEUROSCI.2666-08.2008
Szedlacsek HS et al (2022) Designed peptide inhibitors of STEP Phosphatase-GluA2 AMPA receptor Interaction enhance the cognitive performance in rats. J Med Chem 65(1):217–233. https://doi.org/10.1021/acs.jmedchem.1c01303
Thakkar JP et al (2014) Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 23(10):1985–1996. https://doi.org/10.1158/1055-9965.EPI-14-0275
Ostrom QT et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1-63. https://doi.org/10.1093/neuonc/nou223
Young RM, Jamshidi A, Davis G, Sherman JH (2015) Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med 3(9):121. https://doi.org/10.3978/j.issn.2305-5839.2015.05.10
Louis DN et al (2016) The 2016 World Health Organization classification of tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Tomiyama A, Kobayashi T, Mori K, Ichimura K (2019) Protein phosphatases-a touchy enemy in the battle against glioblastomas: a review. Cancers (Basel) 11(2):E241. https://doi.org/10.3390/cancers11020241
Wang W et al (2011) Crystal structure of human protein tyrosine phosphatase SHP-1 in the open conformation. J Cell Biochem 112(8):2062–2071. https://doi.org/10.1002/jcb.23125
Sooman L et al (2014) PTPN6 expression is epigenetically regulated and influences survival and response to chemotherapy in high-grade gliomas. Tumour Biol 35(5):4479–4488. https://doi.org/10.1007/s13277-013-1590-5
Myers DR et al (2020) Shp1 loss enhances macrophage effector function and promotes Anti-Tumor Immunity. Front Immunol 11:576310. https://doi.org/10.3389/fimmu.2020.576310
Banville D, Ahmad S, Stocco R, Shen SH (1994) A novel protein-tyrosine phosphatase with homology to both the cytoskeletal proteins of the band 4.1 family and junction-associated guanylate kinases. J Biol Chem 269(35):22320–22327
Tajan M, de Rocca Serra A, Valet P, Edouard T, Yart A (2015) SHP2 sails from physiology to pathology. Eur J Med Genet 58(10):509–525. https://doi.org/10.1016/j.ejmg.2015.08.005
Zheng H, Yu W-M, Waclaw RR, Kontaridis MI, Neel BG, Qu C-K (2018) Gain-of-function mutations in the gene encoding the tyrosine phosphatase SHP2 induce hydrocephalus in a catalytically dependent manner. Sci Signal 11(522):eaao1591. https://doi.org/10.1126/scisignal.aao1591
Ostman A, Hellberg C, Böhmer FD (2006) Protein-tyrosine phosphatases and cancer. Nat Rev Cancer 6(4):307–320. https://doi.org/10.1038/nrc1837
Hakak Y, Hsu YS, Martin GS (2000) Shp-2 mediates v-Src-induced morphological changes and activation of the anti-apoptotic protein kinase Akt. Oncogene 19(28):3164–3171. https://doi.org/10.1038/sj.onc.1203655
Ivins Zito C, Kontaridis MI, Fornaro M, Feng G-S, Bennett AM (2004) SHP-2 regulates the phosphatidylinositide 3’-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol 199(2):227–236. https://doi.org/10.1002/jcp.10446
Zhang W et al (2009) Negative regulation of Stat3 by activating PTPN11 mutants contributes to the pathogenesis of Noonan syndrome and juvenile myelomonocytic leukemia. J Biol Chem 284(33):22353–22363. https://doi.org/10.1074/jbc.M109.020495
Chan G, Kalaitzidis D, Neel BG (2008) The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev 27(2):179–192. https://doi.org/10.1007/s10555-008-9126-y
Furcht CM et al (2014) Multivariate signaling regulation by SHP2 differentially controls proliferation and therapeutic response in glioma cells. J Cell Sci 127(Pt 16):3555–3567. https://doi.org/10.1242/jcs.150862
Zhan Y, Counelis GJ, O’Rourke DM (2009) The protein tyrosine phosphatase SHP-2 is required for EGFRvIII oncogenic transformation in human glioblastoma cells. Exp Cell Res 315:2343–2357. https://doi.org/10.1016/j.yexcr.2009.05.001
Song Y, Zhao M, Wu Y, Yu B, Liu H-M (2021) A multifunctional cross-validation high-throughput screening protocol enabling the discovery of new SHP2 inhibitors. Acta Pharm Sin B 11(3):750–762. https://doi.org/10.1016/j.apsb.2020.10.021
Yu K et al (2020) Shp2 activation in bone marrow microenvironment mediates the drug resistance of B-cell acute lymphoblastic leukemia through enhancing the role of VCAM-1/VLA-4. Int Immunopharmacol 80:106008. https://doi.org/10.1016/j.intimp.2019.106008
Song Z et al (2021) Tyrosine phosphatase SHP2 inhibitors in tumor-targeted therapies. Acta Pharm Sin B 11(1):13–29. https://doi.org/10.1016/j.apsb.2020.07.010
Song M et al (2009) NSC-87877, inhibitor of SHP-1/2 PTPs, inhibits dual-specificity phosphatase 26 (DUSP26). Biochem Biophys Res Commun 381(4):491–495. https://doi.org/10.1016/j.bbrc.2009.02.069
Zhou L, Zuo Z, Chow MSS (2005) Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45(12):1345–1359. https://doi.org/10.1177/0091270005282630
Yu X-Y et al (2007) Transport of cryptotanshinone, a major active triterpenoid in Salvia miltiorrhiza Bunge widely used in the treatment of stroke and Alzheimer’s disease, across the blood-brain barrier. Curr Drug Metab 8(4):365–378. https://doi.org/10.2174/138920007780655441
Lu L et al (2017) Cryptotanshinone inhibits human glioma cell proliferation in vitro and in vivo through SHP-2-dependent inhibition of STAT3 activation. Cell Death Dis 8(5):e2767. https://doi.org/10.1038/cddis.2017.174
Wang M, Lu J, Wang M, Yang C-Y, Wang S (2020) Discovery of SHP2-D26 as a First, Potent, and Effective PROTAC Degrader of SHP2 Protein. J Med Chem 63(14):7510–7528. https://doi.org/10.1021/acs.jmedchem.0c00471
Guo W, Xu Q (2020) Phosphatase-independent functions of SHP2 and its regulation by small molecule compounds. J Pharmacol Sci 144(3):139–146. https://doi.org/10.1016/j.jphs.2020.06.002
Y. X et al (2021)Discovery of thalidomide-based PROTAC small molecules as the highly efficient SHP2 degraders. Eur J Med Chem 218. https://doi.org/10.1016/j.ejmech.2021.113341
Zheng M et al (2021) Novel PROTACs for degradation of SHP2 protein. Bioorg Chem 110:104788. https://doi.org/10.1016/j.bioorg.2021.104788
Abaan OD, Levenson A, Khan O, Furth PA, Uren A, Toretsky JA (2005) PTPL1 is a direct transcriptional target of EWS-FLI1 and modulates Ewing’s Sarcoma tumorigenesis. Oncogene 24(16):2715–2722. https://doi.org/10.1038/sj.onc.1208247
Nakai Y, Irie S, Sato TA (2000) Identification of IkappaBalpha as a substrate of Fas-associated phosphatase-1. Eur J Biochem 267(24):7170–7175. https://doi.org/10.1046/j.1432-1327.2000.01818.x
Mcheik S, Aptecar L, Coopman P, D’Hondt V, Freiss G (2020) Dual role of the PTPN13 tyrosine phosphatase in cancer. Biomolecules 10(12):E1659. https://doi.org/10.3390/biom10121659
Nagata S (1994) Apoptosis regulated by a death factor and its receptor: Fas ligand and Fas. Philos Trans R Soc Lond B Biol Sci 345(1313):281–287. https://doi.org/10.1098/rstb.1994.0107
Bompard G, Puech C, Prébois C, Vignon F, Freiss G (2002) Protein-tyrosine phosphatase PTPL1/FAP-1 triggers apoptosis in human breast cancer cells. J Biol Chem 277(49):47861–47869. https://doi.org/10.1074/jbc.M208950200
Ungefroren H et al (2001) FAP-1 in pancreatic cancer cells: functional and mechanistic studies on its inhibitory role in CD95-mediated apoptosis. J Cell Sci 114(Pt 15):2735–2746. https://doi.org/10.1242/jcs.114.15.2735
Foehr ED, Lorente G, Vincent V, Nikolich K, Urfer R (2005) FAS associated phosphatase (FAP-1) blocks apoptosis of astrocytomas through dephosphorylation of FAS. J Neurooncol 74(3):241–248. https://doi.org/10.1007/s11060-004-7202-x
Navis AC, van den Eijnden M, Schepens JTG, Hooft R, van Huijsduijnen P, Wesseling Hendriks WJAJ (2010) Protein tyrosine phosphatases in glioma biology. Acta Neuropathol 119(2):157–175. https://doi.org/10.1007/s00401-009-0614-0
Hampel H et al (2021) The Amyloid-β Pathway in Alzheimer’s Disease. Mol Psychiatry 26(10):5481–5503. https://doi.org/10.1038/s41380-021-01249-0
Braithwaite SP, Stock JB, Lombroso PJ, Nairn AC (2012) Protein phosphatases and Alzheimer’s disease. Prog Mol Biol Transl Sci 106:343–379. https://doi.org/10.1016/B978-0-12-396456-4.00012-2
Wang J-Z, Xia Y-Y, Grundke-Iqbal I, Iqbal K (2013) Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis 33(1):123–139. https://doi.org/10.3233/JAD-2012-129031
Hou T-Y et al (2020) Correcting abnormalities in miR-124/PTPN1 signaling rescues tau pathology in Alzheimer’s disease. J Neurochem 154(4):441–457. https://doi.org/10.1111/jnc.14961
Sanuki R, Yamamura T (2021) Tumor suppressive effects of miR-124 and its function in neuronal development. Int J Mol Sci 22(11):5919. https://doi.org/10.3390/ijms22115919
Wang X et al (2018) A novel MicroRNA-124/PTPN1 signal pathway mediates synaptic and memory deficits in Alzheimer’s Disease. Biol Psychiatry 83(5):395–405. https://doi.org/10.1016/j.biopsych.2017.07.023
Kanno T, Tsuchiya A, Tanaka A, Nishizaki T (2016) Combination of PKCε activation and PTP1B inhibition effectively suppresses Aβ-Induced GSK-3β activation and tau phosphorylation. Mol Neurobiol 53(7):4787–4797. https://doi.org/10.1007/s12035-015-9405-x
Jin N et al (2015) Truncation and activation of GSK-3β by calpain I: a molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci Rep 5:8187. https://doi.org/10.1038/srep08187
Ricke KM et al (2020) Neuronal protein tyrosine phosphatase 1B hastens amyloid β-Associated Alzheimer’s Disease in mice. J Neurosci 40(7):1581–1593. https://doi.org/10.1523/JNEUROSCI.2120-19.2019
Kuga GK et al (2018) Impaired insulin signaling and spatial learning in middle-aged rats: The role of PTP1B. Exp Gerontol 104:66–71. https://doi.org/10.1016/j.exger.2018.02.005
Ghalayini J (2020) Exploring the therapeutic potential of protein tyrosine phosphatase 1B in hAPP-J20 mouse model of Alzheimer’s Disease. J Neurosci 40:6100–6102. https://doi.org/10.1523/JNEUROSCI.0852-20.2020
Liang X, Hou X, Fang H (2021) Structure, function and modulation of Striatal-enriched protein tyrosine phosphatase (STEP). Curr Med Chem 28(37):7714–7728. https://doi.org/10.2174/0929867328666210412123304
Li L, Liang J, Fu H (2021) An update on the association between traumatic brain injury and Alzheimer’s disease: focus on tau pathology and synaptic dysfunction. Neurosci Biobehav Rev 120:372–386. https://doi.org/10.1016/j.neubiorev.2020.10.020
Wang Y, Hall RA, Lee M, Kamgar-parsi A, Bi X, Baudry M (2017) The tyrosine phosphatase PTPN13/FAP-1 links calpain-2, TBI and tau tyrosine phosphorylation. Sci Rep 7(1):11771. https://doi.org/10.1038/s41598-017-12236-3
Santiago JA, Bottero V, Potashkin JA (2018) Evaluation of RNA blood biomarkers in the Parkinson’s disease biomarkers program. Front Aging Neurosci 10:157. https://doi.org/10.3389/fnagi.2018.00157
Feng C-W, Chen N-F, Chan T-F, Chen W-F (2020) Therapeutic role of protein tyrosine phosphatase 1B in Parkinson’s Disease via antineuroinflammation and neuroprotection in vitro and in vivo. Parkinson’s Disease :1–15. https://doi.org/10.1155/2020/8814236
Bend R et al (2020) Phenotype and mutation expansion of the PTPN23 associated disorder characterized by neurodevelopmental delay and structural brain abnormalities. Eur J Hum Genet 28(1):76–87. https://doi.org/10.1038/s41431-019-0487-1
Sowada N et al (2017) Mutations of PTPN23 in developmental and epileptic encephalopathy. Hum Genet 136(11–12):1455–1461. https://doi.org/10.1007/s00439-017-1850-3
Meer Gv, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9(2):112–24. https://doi.org/10.1038/nrm2330
Falabella M, Vernon HJ, Hanna MG, Claypool SM, Pitceathly RDS (2021) Cardiolipin, Mitochondria, and neurological disease. Trends Endocrinol Metab 32(4):224–237. https://doi.org/10.1016/j.tem.2021.01.006
Kagan VE et al (2005) Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1(4):223–232. https://doi.org/10.1038/nchembio727
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Pagliarini DJ et al (2005) Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol Cell 19(2):197–207. https://doi.org/10.1016/j.molcel.2005.06.008
Niemi NM, Lanning NJ, Westrate LM, MacKeigan JP (2013) Downregulation of the mitochondrial phosphatase PTPMT1 is sufficient to promote cancer cell death. PLoS ONE 8(1):e53803. https://doi.org/10.1371/journal.pone.0053803
Zhang J et al (2011) Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab 13(6):690–700. https://doi.org/10.1016/j.cmet.2011.04.007
Acknowledgements
We would like to forward our deepest gratitude to the editors and the anonymous referees for the effort they have invested in reviewing and critiquing our study.
Funding
This work was supported by the National Natural Science Foundation of China. This work was financially supported by the National Natural Science Foundation of China (81602327), and the Zhishan Scholars Programs of Southeast University (2242021R41070).
Author information
Authors and Affiliations
Contributions
YTH and DN collected the materials and wrote the manuscript. HMW designed the research and revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable — no research involving human subjects is included in this manuscript.
Consent for Publication
Not applicable.
Competing Interests
Authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Y., Nan, D. & Wang, H. Role of Non-Receptor-Type Tyrosine Phosphatases in Brain-Related Diseases. Mol Neurobiol 60, 6530–6541 (2023). https://doi.org/10.1007/s12035-023-03487-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03487-5