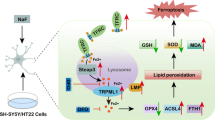
Abstract
Developmental sevoflurane exposure leads to neuronal cell death, and subsequent learning and memory cognitive defects. The underlyi\ng mechanism remains to be elucidated. Gasdermin D (GSDMD)-mediated pyroptosis is a form of inflammatory cell death and participates in a variety of neurodegenerative diseases. Several studies illustrated that dysregulation of mTOR activity is involved in pyroptotic cell death. The current study was designed to interrogate the role of GSDMD-mediated pyroptosis and mTOR activity in developmental sevoflurane exposure. We found that inhibition of GSDMD pore formation with Disulfiram (DSF) or Necrosulfonamide (NSA) significantly attenuated sevoflurane neurotoxicity in vitro. In addition, treatment with DSF or NSA also mitigated damage-associated molecular patterns (DAMPs) release and subsequent plasma membrane rupture (PMR) induced by sevoflurane challenge. Further investigation showed that the overactivation of mTOR signaling is involved in sevoflurane induced pyroptosis both in vivo and in vitro. Intriguingly, we found that the DAMPs release and subsequent PMR triggered by developmental sevoflurane priming were compromised by knocking down the expression of mTORC1 component Raptor, but not mTORC2 component Rictor. Moreover, sevoflurane induced pyroptosis could also be restored by suppressing mTOR activity or knocking down the expressions of Ras-related small GTPases RagA or RagC. Finally, administration of DSF or NSA dramatically improved the spatial and emotional cognitive disorders without alternation of locomotor activity. Taken together, these results indicate that mTORC1-dependent and GSDMD-mediated pyroptosis contributes to the developmental sevoflurane neurotoxicity. Characterizing these processes may provide experimental evidence for the possible prevention of developmental sevoflurane neurotoxicity.








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and are available from the corresponding author on reasonable request.
Abbreviations
- 4E-BP1:
-
EIF-4E binding protein-1
- CNS:
-
Central nervous system
- DAMPs:
-
Damage-associated molecular patterns
- DSF:
-
Disulfiram
- GSDMD:
-
Gasdermin D
- HMGB1:
-
High-mobility group box 1
- LDH:
-
Lactate dehydrogenase
- mTOR:
-
Mammalian target of rapamycin
- MWM:
-
Morris water maze
- NINJ1:
-
Ninjurin-1
- NSA:
-
Necrosulfonamide
- PMR:
-
Plasma membrane rupture
- S6K1:
-
P70 ribosomal protein S6 kinase 1
References
Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z (2010) Anesthetic sevoflurane causes neurotoxicity differently in neonatal naive and Alzheimer disease transgenic mice. Anesthesiology 112:1404–1416
Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110:628–637
Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE et al (2012) Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology 116(3):586–602
Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP (2009) Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 110(4):805–812
DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G (2009) A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol 21:286–291
Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V (2005) Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 135:815–827
Dobbing J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3(1):79–83
Frank D, Vince JE (2019) Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ 26:99–114
Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q et al (2021) NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591:131–136
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S et al (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al (n. d) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660-665
Lammert CR, Frost EL, Bellinger CE, Bolte AC, McKee CA, Hurt ME et al (2020) AIM2 inflammasome surveillance of DNA damage shapes neurodevelopment. Nature 580:647–652
Hu Y, Wang B, Li S, Yang S (2022) Pyroptosis, and its role in central nervous system disease. J Mol Biol 434:167379
Liu GY, Sabatini DM (2020) mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21:183–203
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A et al (n. d) Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83:1131-1143
Pagani M, Barsotti N, Bertero A, Trakoshis S, Ulysse L, Locarno A et al (n. d) mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat Commun 12:6084
Li M-y, Zhu X-l, Zhao B-x, Shi L, Wang W, Hu W et al (2019) Adrenomedullin alleviates the pyroptosis of Leydig cells by promoting autophagy via the ROS–AMPK–mTOR axis. Cell Death Dis 10:1–14
Evavold CL, Hafner-Bratkovic I, Devant P, D’Andrea JM, Ngwa EM, Borsic E et al (2021) Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell 184:4495–4511
Lerman J, Sikich N, Kleinman S, Yentis S (1994) The pharmacology of sevoflurane in infants and children. Anesthesiology 80(4):814–824
Wang WY, Wu XM, Jia LJ, Zhang HH, Cai F, Mao H, Xu WC, Chen L, Zhang J, Hu SF (2016) Beta-arrestin1 and 2 differently modulate metabotropic glutamate receptor 7 signaling in rat developmental sevoflurane-induced neuronal apoptosis. Neuroscience 313:199–212
Wang WY, Jia LJ, Luo Y, Zhang HH, Cai F, Mao H, Xu WC, Fang JB, Peng ZY, Ma ZW, Chen YH, Zhang J, Wei Z, Yu BW, Hu SF (2016) Location-and subunit-specific NMDA receptors determine the developmental sevoflurane neurotoxicity through ERK1/2 signaling. Mol Neurobiol 53:216–230
Wang WY, Wang H, Luo Y, Jia LJ, Zhao JN, Zhang HH, Ma ZW, Xue QS, Yu BW (2012) The effects of metabotropic glutamate receptor 7 allosteric agonist N, N′-dibenzhydrylethane-1, 2-diamine dihydrochloride on developmental sevoflurane neurotoxicity: role of extracellular signal-regulated kinase 1 and 2 mitogen-activated protein kinase signaling pathway. Neuroscience 205:167–177
Wang WY, Luo Y, Jia LJ, Hu SF, Lou XK, Shen SL, Lu H, Zhang HH, Yang R, Wang H, Ma ZW, Xue QS, Yu BW (2014) Inhibition of aberrant cyclin-dependent kinase 5 activity attenuates isoflurane neurotoxicity in the developing brain. Neuropharmacology 77:90–99
Dai J, Li X, Wang C, Gu S, Dai L, Zhang J et al (2021) Repeated neonatal sevoflurane induced neurocognitive impairment through NF-κB-mediated pyroptosis. J Neuroinflamm 18:1–11
Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC (2018) The pore-forming protein gasdermin D regulates Interleukin-1 secretion from living macrophages. Immunity 48:35–44
Russo AJ, Vasudevan SO, Méndez-Huergo SP, Kumari P, Menoret A, Duduskar S et al (2021) Intracellular immune sensing promotes inflammation via gasdermin D–driven release of a lectin alarmin. Nat Immunol 22:154–165
Yang H, Wang H, Andersson U (2020) Targeting inflammation driven by HMGB1. Front Immunol 11:484
Wang Y, Shao F (2021) NINJ1, rupturing swollen membranes for cataclysmic cell lysis. Mol Cell 81(7):1370–1371
Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL et al (2013) Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493:679–683
Ing C, Warner DO, Sun LS, Flick RP, Davidson AJ, Vutskits L et al (2022) Anesthesia and developing brains: unanswered questions and proposed paths forward. Anesthesiology 136(3):500–512
Kolbrink B, Riebeling T, Kunzendorf U, Krautwald S (2020) Plasma membrane pores drive inflammatory. Cell Death Front Cell Dev Biol 8:817
Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B et al (2018) Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science 362:1064–1069
Demarco B, Grayczyk JP, Bjanes E, Le Roy D, Tonnus W, Assenmacher C-A et al (2020) Caspase-8–dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci Adv 6:eabc3465
Zhang H, Zeng L, Xie M, Liu J, Zhou B, Wu R et al (2020) TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe 27:556–570
Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES (2019) Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun 10:1689
Fischer FA, Chen KW, Bezbradica JS (2021) Posttranslational and therapeutic control of gasdermin-mediated pyroptosis and inflammation. Front Immunol 12:661162
Liu X, Xia S, Zhang Z, Wu H, Lieberman J (2021) Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov 20:384–405
Nie Y, Li S, Yan T, Ma Y, Ni C, Wang H, Zheng H (2020) Propofol attenuates isoflurane-induced neurotoxicity and cognitive impairment in fetal and offspring mice. Anesth Analg 131:1616–1625
Tang XL, Wang X, Fang G, Zhao YL, Yan J, Zhou Z et al (2021) Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-kappaB pathway in neonatal mice. J Nutr Biochem 90:108579
Montana MC, Evers AS (2017) Anesthetic neurotoxicity: new findings and future directions. J Pediatr 181:279–285
Paudel YN, Shaikh MF, Chakraborti A, Kumari Y, Aledo-Serrano A, Aleksovska K et al (2018) HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front Neurosci 12:628
Volchuk A, Ye A, Chi L, Steinberg BE, Goldenberg NM (2020) Indirect regulation of HMGB1 release by gasdermin D. Nat Commun 11:4561
Yue J, Hui L, Xie G, Chen S, Wu S, Fang X (2013) Sevoflurane combined with ATP activates caspase-1 and triggers caspase-1-dependent pyroptosis in murine J774 macrophages. Inflammation 36:330–336
Querfurth H, Lee HK (2021) Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener 16:44
Zhang J, Wang C, Yu S, Luo Z, Chen Y, Liu Q et al (2014) Sevoflurane postconditioning protects rat hearts against ischemia-reperfusion injury via the activation of PI3K/AKT/mTOR signaling. Sci Rep 4:7317
Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC et al (2018) Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 3:eaat2738
Sun L, Wang H, Wang Z, He S, Chen S, Liao D et al (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227
Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J et al (2020) FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol 21:736–745
Wang C, Yang T, Xiao J, Xu C, Mbalaviele G (2021) Activation of GSDME compensates for GSDMD deficiency in a mouse model of NLRP3 inflammasomopathy. bioRxiv 2021 https://doi.org/10.1101/2021.01.06.425634
Wang Y, Gao W, Shi X, Ding J, Liu W, He H et al (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547:99–103
Taabazuing CY, Okondo MC, Bachovchin DA (2017) Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol 24:507–514
Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M et al (2012) HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med 18:930–937
Brück E, Lasselin J, Andersson U, Sackey PV, Olofsson PS (2020) Prolonged elevation of plasma HMGB1 is associated with cognitive impairment in intensive care unit survivors. Intensive Care Med 46:811–812
Kang E, Jiang D, Ryu YK, Lim S, Kwak M, Gray CD et al (2017) Early postnatal exposure to isoflurane causes cognitive deficits and disrupts development of newborn hippocampal neurons via activation of the mTOR pathway. PLoS Biol 15:e2001246
Wen J, Xu J, Mathena RP, Choi JH, Mintz CD (2021) Early isoflurane exposure impairs synaptic development in Fmr1 KO mice via the mTOR pathway. Neurochem Res 46:1577–1588
Vanderplow AM, Eagle AL, Kermath BA, Bjornson KJ, Robison AJ, Cahill ME (2021) Akt-mTOR hypoactivity in bipolar disorder gives rise to cognitive impairments associated with altered neuronal structure and function. Neuron 109:1479–1496
Bedoui S, Herold MJ, Strasser A (2020) Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol 21:678–695
de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M (2019) Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ 26:146–161
Acknowledgements
The authors wish to thank Luo Fo-Quan and Hong Hua-li for critical comments on this topic.
Funding
This research was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LY18H310007 (Wen-Yuan Wang), and the National Natural Science Foundation, Beijing, China, grant No. 81302858 (Wen-Yuan Wang), the National Key Research and Development Program of China (No. 2018YFC2001904) and the Zhejiang Provincial Science and Technology Project (No. 2023574725).
Author information
Authors and Affiliations
Contributions
WWY designed the study, conducted the majority of the experiments and wrote most of the manuscript. YWQ prepared neuronal cultures and wrote the preliminary manuscript. HQY and LYS helped with western blot analysis. QSJ and LJT performed the behavior study. MH and CF carried out FCM and immunofluorescence. YHL performed confocal microscopy, analyzed the results, edited the manuscript and contributed to the supervision of the study. All authors approved the version to be published.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments and protocol have been approved by the Animal Care and Use Committee of Zhejiang Provincial People’s Hospital (Affiliated People`s Hospital, Hangzhou Medical College).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen-Yuan, W., Wan-Qing, Y., Qi-Yun, H. et al. mTORC1-Dependent and GSDMD-Mediated Pyroptosis in Developmental Sevoflurane Neurotoxicity. Mol Neurobiol 60, 116–132 (2023). https://doi.org/10.1007/s12035-022-03070-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03070-4