Abstract
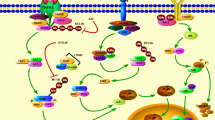
Spinal cord injury (SCI) is a devastating neurological disorder that usually damages sensorimotor and autonomic functions. Signaling pathways can play a key role in the repair process of SCI. The plexin-B2 acts as a receptor for angiogenin and mediates ribosomal RNA transcription, influencing cell survival and proliferation. Protein kinase B serine/threonine kinase interacts with angiogenin to form a positive feedback effect. Brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor can induce angiogenin nuclear translocation. Moreover, the BDNF can promote the secretion of angiogenin. Interestingly, all of them can activate the angiogenin/plexin-B2 axis. Muscone has anti-inflammatory and proliferative features as it can inhibit nuclear transcription factor kappa-B (NF-κB) and activate the angiogenin/plexin-B2 axis, thus being significant agent in the SCI repair process. Herein, we review the potential mechanism of angiogenin/plexin-B2 axis activation and the role of muscone in SCI treatment. Muscone may attenuate inflammatory responses and promote neuronal regeneration after SCI.

Similar content being viewed by others
Data Availability
Not applicable.
References
Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG (2017) Traumatic spinal cord injury. Nat Rev Dis Primers 3:17018. https://doi.org/10.1038/nrdp.2017.18
Badhiwala JH, Wilson JR, Fehlings MG (2019) Global burden of traumatic brain and spinal cord injury. Lancet Neurol 18(1):24–25. https://doi.org/10.1016/S1474-4422(18)30444-7
GBD 2016 Neurology Collaborators (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18(5):459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK et al (2020) Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 21(20):7533. https://doi.org/10.3390/ijms21207533
Okada S (2016) The pathophysiological role of acute inflammation after spinal cord injury. Inflamm Regen 36:20. https://doi.org/10.1186/s41232-016-0026-1
Al Mamun A, Wu Y, Monalisa I, Jia C, Zhou K, Munir F, Xiao J (2020) Role of pyroptosis in spinal cord injury and its therapeutic implications. J Adv Res 28:97–109. https://doi.org/10.1016/j.jare.2020.08.004
Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B (2018) hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl 89:194–204. https://doi.org/10.1016/j.msec.2018.04.006
Hu XC, Lu YB, Yang YN, Kang XW, Wang YG, Ma B, Xing S (2021) Progress in clinical trials of cell transplantation for the treatment of spinal cord injury: how many questions remain unanswered? Neural Regen Res 16(3):405–413. https://doi.org/10.4103/1673-5374.293130
Yang Y, Fan Y, Zhang H, Zhang Q, Zhao Y, Xiao Z, Liu W, Chen B et al (2021) Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 269:120479. https://doi.org/10.1016/j.biomaterials.2020.120479
Lv R, Du L, Zhang L, Zhang Z (2019) Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci 217:119–127. https://doi.org/10.1016/j.lfs.2018.11.053
Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, Zachariou V, Guo L et al (2020) Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat Neurosci 23(3):337–350. https://doi.org/10.1038/s41593-020-0597-7
Yu W, Goncalves KA, Li S, Kishikawa H, Sun G, Yang H, Vanli N, Wu Y et al (2017) Plexin-B2 mediates physiologic and pathologic functions of angiogenin. Cell 171(4):849-864.e25. https://doi.org/10.1016/j.cell.2017.10.005
Phung HM, Lee S, Hwang JH, Kang KS (2020) preventive effect of muscone against cisplatin nephrotoxicity in LLC-PK1 cells. Biomolecules 10(10):1444. https://doi.org/10.3390/biom10101444
Zhou LY, Yao M, Tian ZR, Liu SF, Song YJ, Ye J, Li G, Sun YL et al (2020) Muscone suppresses inflammatory responses and neuronal damage in a rat model of cervical spondylotic myelopathy by regulating Drp1-dependent mitochondrial fission. J Neurochem 155(2):154–176. https://doi.org/10.1111/jnc.15011
Abd El Wahab MG, Ali SS, Ayuob NN (2018) The role of musk in relieving the neurodegenerative changes induced after exposure to chronic stress. Am J Alzheimers Dis Other Demen. 33(4):221–231. https://doi.org/10.1177/1533317518755993
Mori A, Nishioka Y, Yamada M, Nishibata Y, Masuda S, Tomaru U, Honma N, Moriyama T et al (2018) Brain-derived neurotrophic factor induces angiogenin secretion and nuclear translocation in human umbilical vein endothelial cells. Pathol Res Pract 214(4):521–526. https://doi.org/10.1016/j.prp.2018.02.013
Du Y, Ge Y, Xu Z, Aa N, Gu X, Meng H, Lin Z, Zhu D et al (2018) Hypoxia-inducible factor 1 alpha (HIF-1α)/vascular endothelial growth factor (VEGF) pathway participates in angiogenesis of myocardial infarction in muscone-treated mice: preliminary study. Med Sci Monit 24:8870–8877. https://doi.org/10.12659/MSM.912051
Karki A, Purohit R, Nosack S, Bharathy N, Michalek JE, Chen S, Keller C (2022) Plexin-B2 and semaphorins do not drive rhabdomyosarcoma proliferation or migration. Sarcoma 2022:9646909. https://doi.org/10.1155/2022/9646909
Hemida AS, Mareae AH, Elbasiony ASA, Shehata WA (2020) Plexin-B2 in psoriasis; a clinical and immunohistochemical study. J Immunoassay Immunochem 41(4):718–728. https://doi.org/10.1080/15321819.2020.1741385
Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML et al (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99(1):71–80. https://doi.org/10.1016/s0092-8674(00)80063-x
Deng S, Hirschberg A, Worzfeld T, Penachioni JY, Korostylev A, Swiercz JM, Vodrazka P, Mauti O et al (2007) Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo. J Neurosci 27(23):6333–6347. https://doi.org/10.1523/JNEUROSCI.5381-06.2007
Nishide M, Kumanogoh A (2018) The role of semaphorins in immune responses and autoimmune rheumatic diseases. Nat Rev Rheumatol 14(1):19–31. https://doi.org/10.1038/nrrheum.2017.201
McDermott JE, Goldblatt D, Paradis S (2018) Class 4 Semaphorins and Plexin-B receptors regulate GABAergic and glutamatergic synapse development in the mammalian hippocampus. Mol Cell Neurosci 92:50–66. https://doi.org/10.1016/j.mcn.2018.06.008
Simonetti M, Paldy E, Njoo C, Bali KK, Worzfeld T, Pitzer C, Kuner T, Offermanns S et al (2021) The impact of semaphorin 4C/plexin-B2 signaling on fear memory via remodeling of neuronal and synaptic morphology. Mol Psychiatry 26(4):1376–1398. https://doi.org/10.1038/s41380-019-0491-4
Van Battum E, Heitz-Marchaland C, Zagar Y, Fouquet S, Kuner R, Chédotal A (2021) Plexin-B2 controls the timing of differentiation and the motility of cerebellar granule neurons. Elife 10:e60554. https://doi.org/10.7554/eLife.60554
Xia J, Swiercz JM, Bañón-Rodríguez I, Matković I, Federico G, Sun T, Franz T, Brakebusch CH et al (2015) Semaphorin-plexin signaling controls mitotic spindle orientation during epithelial morphogenesis and repair. Dev Cell 33(3):299–313. https://doi.org/10.1016/j.devcel.2015.02.001
Paldy E, Simonetti M, Worzfeld T, Bali KK, Vicuña L, Offermanns S, Kuner R (2017) Semaphorin 4C Plexin-B2 signaling in peripheral sensory neurons is pronociceptive in a model of inflammatory pain. Nat Commun 8(1):176. https://doi.org/10.1038/s41467-017-00341-w
Povysheva T, Shmarov M, Logunov D, Naroditsky B, Shulman I, Ogurcov S, Kolesnikov P, Islamov R et al (2017) Post-spinal cord injury astrocyte-mediated functional recovery in rats after intraspinal injection of the recombinant adenoviral vectors Ad5-VEGF and Ad5-ANG. J Neurosurg Spine 27(1):105–115. https://doi.org/10.3171/2016.9.SPINE15959
Bai R, Sun D, Chen M, Shi X, Luo L, Yao Z, Liu Y, Ge X et al (2020) Myeloid cells protect intestinal epithelial barrier integrity through the angiogenin/plexin-B2 axis. EMBO J 39(13):e103325. https://doi.org/10.15252/embj.2019103325
Oikonomou KA, Kapsoritakis AN, Kapsoritaki AI, Manolakis AC, Tiaka EK, Tsiopoulos FD, Tsiompanidis IA, Potamianos SP (2011) Angiogenin, angiopoietin-1, angiopoietin-2, and endostatin serum levels in inflammatory bowel disease. Inflamm Bowel Dis 17(4):963–970. https://doi.org/10.1002/ibd.21410
Junqueira Alves C, Dariolli R, Haydak J, Kang S, Hannah T, Wiener RJ, DeFronzo S, Tejero R et al (2021) Plexin-B2 orchestrates collective stem cell dynamics via actomyosin contractility, cytoskeletal tension and adhesion. Nat Commun. 12(1):6019. https://doi.org/10.1038/s41467-021-26296-7
Xiao C, Luo Y, Zhang C, Zhu Z, Yang L, Qiao H, Fu M, Wang G et al (2020) Negative regulation of dendritic cell activation in psoriasis mediated via CD100-plexin-B2. J Pathol 250(4):409–419. https://doi.org/10.1002/path.5383
Daviaud N, Chen K, Huang Y, Friedel RH, Zou H (2016) Impaired cortical neurogenesis in plexin-B1 and -B2 double deletion mutant. Dev Neurobiol 76(8):882–899. https://doi.org/10.1002/dneu.22364
Zhang Y, Shen S, Li P, Fan Y, Zhang L, Li W, Liu Y (2019) PLEXIN-B2 promotes the osteogenic differentiation of human bone marrow mesenchymal stem cells via activation of the RhoA signaling pathway. Cell Signal 62:109343. https://doi.org/10.1016/j.cellsig.2019.06.008
Garcia A, Dunoyer-Geindre S, Zapilko V, Nolli S, Reny JL, Fontana P (2019) Functional validation of microrna-126-3p as a platelet reactivity regulator using human haematopoietic stem cells. Thromb Haemost 119(2):254–263. https://doi.org/10.1055/s-0038-1676802
Atkin-Smith GK, Miles MA, Tixeira R, Lay FT, Duan M, Hawkins CJ, Phan TK, Paone S et al (2019) Plexin B2 is a regulator of monocyte apoptotic cell disassembly. Cell Rep 29(7):1821-1831.e3. https://doi.org/10.1016/j.celrep.2019.10.014
Yan H, Wu L, Shih C, Hou S, Shi J, Mao T, Chen W, Melvin B et al (2017) Plexin B2 and semaphorin 4C guide T cell recruitment and function in the germinal center. Cell Rep 19(5):995–1007. https://doi.org/10.1016/j.celrep.2017.04.022
Zhang C, Xiao C, Dang E, Cao J, Zhu Z, Fu M, Yao X, Liu Y et al (2018) CD100-Plexin-B2 promotes the inflammation in psoriasis by activating NF-κB and the inflammasome in keratinocytes. J Invest Dermatol 138(2):375–383. https://doi.org/10.1016/j.jid.2017.09.005
Nishide M, Nojima S, Ito D, Takamatsu H, Koyama S, Kang S, Kimura T, Morimoto K et al (2017) Semaphorin 4D inhibits neutrophil activation and is involved in the pathogenesis of neutrophil-mediated autoimmune vasculitis. Ann Rheum Dis 76(8):1440–1448. https://doi.org/10.1136/annrheumdis-2016-210706
Gensel JC, Zhang B (2015) Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res 1619:1–11. https://doi.org/10.1016/j.brainres.2014.12.045
Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133(Pt 2):433–447. https://doi.org/10.1093/brain/awp322
Zhou Y, Dong Q, Pan Z, Song Y, Su P, Niu Y, Sun Y, Liu D (2019) hyperbaric oxygen improves functional recovery of the injured spinal cord by inhibiting inflammation and glial scar formation. Am J Phys Med Rehabil 98(10):914–920. https://doi.org/10.1097/PHM.0000000000001225
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532(7598):195–200. https://doi.org/10.1038/nature17623
Wang C, Wang Q, Lou Y, Xu J, Feng Z, Chen Y, Tang Q, Zheng G et al (2018) Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med 22(2):1148–1166. https://doi.org/10.1111/jcmm.13368
Li Y, He X, Kawaguchi R, Zhang Y, Wang Q, Monavarfeshani A, Yang Z, Chen B et al (2020) Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 587(7835):613–618. https://doi.org/10.1038/s41586-020-2795-6
Chen G, Li J, Wang Z, Liu W (2020) Ezetimibe protects against spinal cord injury by regulating autophagy and apoptosis through inactivation of PI3K/AKT/mTOR signaling. Am J Transl Res 12(6):2685–2694
Song M, Bode AM, Dong Z, Lee MH (2019) AKT as a therapeutic target for cancer. Cancer Res 79(6):1019–1031. https://doi.org/10.1158/0008-5472.CAN-18-2738
Revathidevi S, Munirajan AK (2019) Akt in cancer: Mediator and more. Semin Cancer Biol 59:80–91. https://doi.org/10.1016/j.semcancer.2019.06.002
Zeng Y, Du WW, Wu Y, Yang Z, Awan FM, Li X, Yang W, Zhang C et al (2017) A Circular RNA Binds To and Activates AKT Phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics 7(16):3842–3855. https://doi.org/10.7150/thno.19764
Datta SR, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13(22):2905–2927. https://doi.org/10.1101/gad.13.22.2905
Umar S, Soni R, Durgapal SD, Soman S, Balakrishnan S (2020) A synthetic coumarin derivative (4-flourophenylacetamide-acetyl coumarin) impedes cell cycle at G0/G1 stage, induces apoptosis, and inhibits metastasis via ROS-mediated p53 and AKT signaling pathways in A549 cells. J Biochem Mol Toxicol 34(10):e22553. https://doi.org/10.1002/jbt.22553
He Y, Liu X, Chen Z (2020) Glial scar-a promising target for improving outcomes after CNS injury. J Mol Neurosci 70(3):340–352. https://doi.org/10.1007/s12031-019-01417-6
Hart CG, Karimi-Abdolrezaee S (2021) Recent insights on astrocyte mechanisms in CNS homeostasis, pathology, and repair. J Neurosci Res 99(10):2427–2462. https://doi.org/10.1002/jnr.24922
Xu X, Zhang A, Zhu Y, He W, Di W, Fang Y, Shi X (2018) MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. J Cell Physiol 234(1):904–914. https://doi.org/10.1002/jcp.26918
Ju WK, Shim MS, Kim KY, Park TL, Ahn S, Edwards G, Weinreb RN (2019) Inhibition of cAMP/PKA pathway protects optic nerve head astrocytes against oxidative stress by Akt/Bax phosphorylation-mediated Mfn1/2 oligomerization. Oxid Med Cell Longev 2019:8060962. https://doi.org/10.1155/2019/8060962
Gao Q, Yu G, Yu M, Wang X (2020) Bupleuri radix prevents the recurrences of resected colonic polyps by affecting angiogenin-2-induced protein kinase B/Akt signaling. J Oncol 2020:3531652. https://doi.org/10.1155/2020/3531652
Kim HM, Kang DK, Kim HY, Kang SS, Chang SI (2007) Angiogenin-induced protein kinase B/Akt activation is necessary for angiogenesis but is independent of nuclear translocation of angiogenin in HUVE cells. Biochem Biophys Res Commun 352(2):509–513. https://doi.org/10.1016/j.bbrc.2006.11.047
Huang F, Chen T, Chang J, Zhang C, Liao F, Wu L, Wang W, Yin Z (2021) A conductive dual-network hydrogel composed of oxidized dextran and hyaluronic-hydrazide as BDNF delivery systems for potential spinal cord injury repair. Int J Biol Macromol 167:434–445. https://doi.org/10.1016/j.ijbiomac.2020.11.206
Masuda K, Furuyama T, Takahara M, Fujioka S, Kurinami H, Inagaki S (2004) Sema4D stimulates axonal outgrowth of embryonic DRG sensory neurones. Genes Cells 9(9):821–829. https://doi.org/10.1111/j.1365-2443.2004.00766.x
Kailiang Z, Yihui Z, Dingsheng L, Xianyao T (2016) effects of muscone on random skin flap survival in Rats. J Reconstr Microsurg 32(3):200–207. https://doi.org/10.1055/s-0035-1565264
Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF (2005) Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 24(3):445–456. https://doi.org/10.1038/sj.onc.1208223
He Y, Li Z, Chen Z, Yu X, Ji Z, Wang J, Qian Y, Li L (2018) Effects of VEGF-ANG-1-PLA nano-sustained release microspheres on proliferation and differentiation of ADSCs. Cell Biol Int 42(8):1060–1068. https://doi.org/10.1002/cbin.10986
Wang J, Xing H, Qin X, Ren Q, Yang J, Li L (2020) Pharmacological effects and mechanisms of muscone. J Ethnopharmacol 262:113120. https://doi.org/10.1016/j.jep.2020.113120
Liu Y, Bian H, Xu S, Shu S, Jia J, Chen J, Cao X, Bao X et al (2020) muscone ameliorates synaptic dysfunction and cognitive deficits in APP/PS1 mice. J Alzheimers Dis 76(2):491–504. https://doi.org/10.3233/JAD-200188
He MC, Shi Z, Qin M, Sha NN, Li Y, Liao DF, Lin FH, Shu B et al (2020) Muscone ameliorates LPS-induced depressive-like behaviors and inhibits neuroinflammation in prefrontal cortex of mice. Am J Chin Med 48(3):559–577. https://doi.org/10.1142/S0192415X20500287
Yu S, Zhao G, Han F, Liang W, Jiao Y, Li Z, Li L (2020) Muscone relieves inflammatory pain by inhibiting microglial activation-mediated inflammatory response via abrogation of the NOX4/JAK2-STAT3 pathway and NLRP3 inflammasome. Int Immunopharmacol 82:106355. https://doi.org/10.1016/j.intimp.2020.106355
Yu L, Wang N, Zhang Y, Wang Y, Li J, Wu Q, Liu Y (2014) Neuroprotective effect of muscone on glutamate-induced apoptosis in PC12 cells via antioxidant and Ca(2+) antagonism. Neurochem Int 70:10–21. https://doi.org/10.1016/j.neuint.2014.03.003
Liu Z, Li H, Ma W, Pan S (2021) Network pharmacology to investigate the pharmacological mechanisms of muscone in Xingnaojing injections for the treatment of severe traumatic brain injury. PeerJ 9:e11696. https://doi.org/10.7717/peerj.11696
Liu K, Xie L, Deng M, Zhang X, Luo J, Li X (2021) Zoology, chemical composition, pharmacology, quality control and future perspective of Musk (Moschus): a review. Chin Med 16(1):46. https://doi.org/10.1186/s13020-021-00457-8
Wei CJ, Hua F, Chen YH, Zhang ZW, Shen ZY (2021) Muscone alleviates myocardial ischemia-reperfusion injury via inhibition of oxidative stress and enhancement of SIRT3. J Biol Regul Homeost Agents 35(1):85–96. https://doi.org/10.23812/20-101-A
Guo L, Quan ZX, Zhao ZH, Tang K, Ou YS, Jiang DM (2015) Effects of musk ketone on nerve recovery after spinal cord injury. Genet Mol Res 14(2):2958–2963. https://doi.org/10.4238/2015.April.10.4
David S, López-Vales R, Wee YV (2012) Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb Clin Neurol 109:485–502. https://doi.org/10.1016/B978-0-444-52137-8.00030-9
Xiyang YB, Lu BT, Ya-Zhao, Yuan-Zhang, Xia QJ, Zou Y, Zhang W, Quan XZ et al (2014) Expressional difference, distributions of TGF-β1 in TGF-β1 knock down transgenic mouse, and its possible roles in injured spinal cord. Exp Biol Med (Maywood). 239(3):320–9. https://doi.org/10.1177/1535370213509562
Park J, Choi H, Min JS, Park SJ, Kim JH, Park HJ, Kim B, Chae JI et al (2013) Mitochondrial dynamics modulate the expression of pro-inflammatory mediators in microglial cells. J Neurochem 127(2):221–232. https://doi.org/10.1111/jnc.12361
Kang S, Duan W, Zhang S, Chen D, Feng J, Qi N (2020) Muscone/RI7217 co-modified upward messenger DTX liposomes enhanced permeability of blood-brain barrier and targeting glioma. Theranostics 10(10):4308–4322. https://doi.org/10.7150/thno.41322
Goncalves KA, Silberstein L, Li S, Severe N, Hu MG, Yang H, Scadden DT, Hu GF (2016) Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell 166(4):894–906. https://doi.org/10.1016/j.cell.2016.06.042
Li S, Goncalves KA, Lyu B, Yuan L, Hu GF (2020) Chemosensitization of prostate cancer stem cells in mice by angiogenin and plexin-B2 inhibitors. Commun Biol 3(1):26. https://doi.org/10.1038/s42003-020-0750-6
Liu X, Chai Y, Liu G, Su W, Guo Q, Lv X, Gao P, Yu B et al (2021) Osteoclasts protect bone blood vessels against senescence through the angiogenin/plexin-B2 axis. Nat Commun 12(1):1832. https://doi.org/10.1038/s41467-021-22131-1
Dong J, Li H, Bai Y, Wu C (2019) Muscone ameliorates diabetic peripheral neuropathy through activating AKT/mTOR signalling pathway. J Pharm Pharmacol 71(11):1706–1713. https://doi.org/10.1111/jphp.13157
Wang X, Meng H, Chen P, Yang N, Lu X, Wang ZM, Gao W, Zhou N et al (2014) Beneficial effects of muscone on cardiac remodeling in a mouse model of myocardial infarction. Int J Mol Med 34(1):103–111. https://doi.org/10.3892/ijmm.2014.1766
Jin Z, Cheng X, Feng H, Kuang J, Yang W, Peng C, Shen B, Qiu W (2017) Apatinib inhibits angiogenesis via suppressing Akt/GSK3β/ANG signaling pathway in anaplastic thyroid cancer. Cell Physiol Biochem 44(4):1471–1484. https://doi.org/10.1159/000485583
Peng Y, Li L, Huang M, Duan C, Zhang L, Chen J (2014) Angiogenin interacts with ribonuclease inhibitor regulating PI3K/AKT/mTOR signaling pathway in bladder cancer cells. Cell Signal 26(12):2782–2792. https://doi.org/10.1016/j.cellsig.2014.08.021
Zhai X, Yan Z, Zhao J, Chen K, Yang Y, Cai M, He C, Huang C et al (2020) Muscone ameliorates ovariectomy-induced bone loss and receptor activator of nuclear factor-κb ligand-induced osteoclastogenesis by suppressing TNF receptor-associated factor 6-mediated signaling pathways. Front Pharmacol 11:348. https://doi.org/10.3389/fphar.2020.00348
Si YC, Li Q, Xie CE, Niu X, Xia XH, Yu CY (2014) Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chin Med 9(1):13. https://doi.org/10.1186/1749-8546-9-13
Gabriel-Salazar M, Lei T, Grayston A, Costa C, Medina-Gutiérrez E, Comabella M, Montaner J, Rosell A (2021) Angiogenin in the neurogenic subventricular zone after stroke. Front Neurol 12:662235. https://doi.org/10.3389/fneur.2021.662235
Yang H, Yuan L, Ibaragi S, Li S, Shapiro R, Vanli N, Goncalves KA, Yu W et al (2022) Angiogenin and plexin-B2 axis promotes glioblastoma progression by enhancing invasion, vascular association, proliferation and survival. Br J Cancer. https://doi.org/10.1038/s41416-022-01814-6
Funding
The Natural Science Foundation of Zhejiang Province supported this work (no. LY19H170001).
Author information
Authors and Affiliations
Contributions
XL designed the study. YZ, SG, YZ, BOAB, TJ, and XL prepared the first draft of the manuscript. YZ, SG, YZ, BOAB, and XL revised the manuscript. All authors approved the final paper.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors agreed to publish this article.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Guo, S., Botchway, B.O.A. et al. Muscone Can Improve Spinal Cord Injury by Activating the Angiogenin/Plexin-B2 Axis. Mol Neurobiol 59, 5891–5901 (2022). https://doi.org/10.1007/s12035-022-02948-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02948-7