Abstract
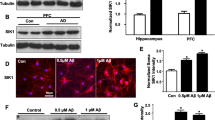
Dehydroeffusol, a phenanthrene isolated from Juncus effusus, is a Chinese medicine. To explore an efficacy of dehydroeffusol administration for prevention and cure of Alzheimer’s disease, here we examined the effect of dehydroeffusol on amyloid β1-42 (Aβ1-42)-mediated hippocampal neurodegeneration. Dehydroeffusol (15 mg/kg body weight) was orally administered to mice once a day for 6 days and then human Aβ1-42 was injected intracerebroventricularly followed by oral administration for 12 days. Neurodegeneration in the dentate granule cell layer, which was determined 2 weeks after Aβ1-42 injection, was rescued by dehydroeffusol administration. Aβ staining (uptake) was not reduced in the dentate granule cell layer by pre-administration of dehydroeffusol for 6 days, while increase in intracellular Zn2+ induced with Aβ1-42 was reduced, suggesting that pre-administration of dehydroeffusol prior to Aβ1-42 injection is effective for Aβ1-42-mediated neurodegeneration that was linked with intracellular Zn2+ toxicity. As a matter of fact, pre-administration of dehydroeffusol rescued Aβ1-42-mediated neurodegeneration. Interestingly, pre-administration of dehydroeffusol increased synthesis of metallothioneins, intracellular Zn2+-binding proteins, in the dentate granule cell layer, which can capture Zn2+ from Zn-Aβ1-42 complexes. The present study indicates that pre-administration of dehydroeffusol protects Aβ1-42-mediated neurodegeneration in the hippocampus by reducing intracellular Zn2+ toxicity, which is linked with induced synthesis of metallothioneins. Dehydroeffusol, a novel inducer of metallothioneins, may protect Aβ1-42-induced pathogenesis in Alzheimer’s disease.








Similar content being viewed by others
Data Availability
Not applicable
References
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601
Knierim JJ (2015) The hippocampus. Curr Biol 25:R1116–R1121
Sasaki T, Leutgeb S, Leutgeb JK (2015) Spatial and memory circuits in the medial entorhinal cortex. Curr Opin Neurobiol 32:16–23
Moryś J, Sadowski M, Barcikowska M, Maciejewska B, Narkiewicz O (1994) The second layer neurones of the entorhinal cortex and the perforant path in physiological ageing and Alzheimer’s disease. Acta Neurobiol Exp (Wars) 54:47–53
Gómez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16:4491–4500
Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, Kim D, Hamto P et al (2010) Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron 68:428–441
Qin Y, Tian Y, Han H, Liu L, Ge X, Xue H, Wang T, Zhou L et al (2019) Risk classification for conversion from mild cognitive impairment to Alzheimer’s disease in primary care. Psychiatry Res 278:19–26
Nestor PJ, Scheltens P, Hodges JR (2004) Advances in the early detection of Alzheimer’s disease. Nat Med 10:S34–S41
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344
Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM et al (2005) Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48:913–922
Morys J, Bobinski M, Wegiel J, Wisniewski HM, Narkiewicz O (1996) Alzheimer’s disease severely affects areas of the claustrum connected with the entorhinal cortex. J Hirnforsch 37:173–180
Funato H, Yoshimura M, Kusui K, Tamaoka A, Ishikawa K, Ohkoshi N, Namekata K, Okeda R et al (1998) Quantitation of amyloid beta-protein (A beta) in the cortex during aging and in Alzheimer’s disease. Am J Pathol 152:1633–1640
Takeda A, Tamano H, Tempaku M, Sasaki M, Uematsu C, Sato S, Kanazawa H, Datki ZL et al (2017) Extracellular Zn2+ is essential for amyloid β1-42-induced cognitive decline in the normal brain and its rescue. J Neurosci 37:7253–7262
Tamano H, Oneta N, Shioya A, Adlard PA, Bush AI, Takeda A (2019) In vivo synaptic activity-independent co-uptakes of amyloid β1-42 and Zn2+ into dentate granule cells in the normal brain. Sci Rep 9:6498
Crichton GE, Bryan J, Murphy KJ (2013) Dietary antioxidants, cognitive function and dementia--a systematic review. Plant Foods Hum Nutr 68:279–292
Liao YJ, Zhai HF, Zhang B, Duan TX, Huang JM (2011) Anxiolytic and sedative effects of dehydroeffusol from Juncus effusus in mice. Planta Med 77:416–420
Wang Y, Wang Y, Zhai H, Liao Y, Zhang B, Huang J (2012) Phenanthrenes from Juncus effusus with anxiolytic and sedative activities. Nat Prod Res 26:1234–1239
Greca MD, Fiorentino A, Monaco P, Pinto G, Pollio A, Previtera L (1996) Action of antialgal compounds from Juncus effusus L. on Selenastrum capricornutum. J Chem Ecol 22:587–603
Singhuber J, Baburin I, Khom S, Zehl M, Urban E, Hering S, Kopp B (2012) GABA(A) receptor modulators from the Chinese herbal drug Junci Medulla--the pith of Juncus effusus. Planta Med 78:455–458
Fukuda T, Sato Y, Takiguchi M, Yamamoto T, Murasawa H, Pawlak A, Kobayashi H, Tamano H et al (2020) Dehydroeffusol rescues amyloid β25-35-induced spatial working memory deficit. Plant Foods Hum Nutr 75:279–282
Tamano H, Takiguchi M, Tanaka Y, Murakami T, Adlard PA, Bush AI, Takeda A (2020) Preferential neurodegeneration in the dentate gyrus by amyloid β1-42-induced intracellular Zn2+ dysregulation and its defense strategy. Mol Neurobiol 57:1875–1888
Takeda A, Tamano H, Hashimoto W, Kobuchi S, Suzuki H, Murakami T, Tempaku M, Koike Y et al (2018) Novel defense by metallothionein induction against cognitive decline: from amyloid β1-42-induced excess Zn2+ to functional Zn2+ deficiency. Mol Neurobiol 55:7775–7788
Sato Y, Takiguchi M, Tamano H, Takeda A (2021) Extracellular Zn2+-dependent amyloid-β1-42 neurotoxicity in Alzheimer’s disease pathogenesis. Biol Trace Elem Res 199:53–61
Abramovitch-Dahan C, Asraf H, Bogdanovic M, Sekler I, Bush AI, Hershfinkel M (2016) Amyloid β attenuates metabotropic zinc sensing receptor, mZnR/GPR39, dependent Ca2+, ERK1/2 and Clusterin signaling in neurons. J Neurochem 139:221–233
Haq F, Mahoney M, Koropatnick J (2003) Signaling events for metallothionein induction. Mutat Res 533:211–216
Ebadi M, Iversen PL, Hao R, Cerutis DR, Rojas P, Happe HK, Murrin LC, Pfeiffer RF (1995) Expression and regulation of brain metallothionein. Neurochem Int 27:1–22
Krężel A, Maret W (2006) Zinc buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem 11:1049–1062
Krężel A, Hao Q, Maret W (2007) The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys 463:188–200
Krężel A, Maret W (2007) Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J Am Chem Soc 129:10911–10921
Krężel A, Maret W (2017) The functions of metamorphic metallothioneins in zinc and copper metabolism. Int J Mol Sci 18. https://doi.org/10.3390/ijms18061237
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y, Balaji RV, Masalha R et al (2006) Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol 198:285–293
Vasák M, Kägi JH (1983) Spectroscopic properties of metallothionein. In: Sigel H (ed) Metal Ions in Biological Systems, vol 15. Marcel Dekker, New York, pp. 213–273
Tamano H, Takiguchi M, Shimaya R, Adlard PA, Bush AI, Takeda A (2019) Extracellular Zn2+-independently attenuated LTP by human amyloid β1-40 and rat amyloid β1-42. Biochem Biophys Res Commun 514:888–892
de Ceballos ML, Brera B, Fernández-Tomé MP (2001) beta-Amyloid-induced cytotoxicity, peroxide generation and blockade of glutamate uptake in cultured astrocytes. Clin Chem Lab Med 39:317–318
Gray CW, Patel AJ (1995) Neurodegeneration mediated by glutamate and beta-amyloid peptide: a comparison and possible interaction. Brain Res 691:169–179
Gilad D, Shorer S, Ketzef M, Friedman A, Sekler I, Aizenman E, Hershfinkel M (2015) Homeostatic regulation of KCC2 activity by the zinc receptor mZnR/GPR39 during seizures. Neurobiol Dis 81:4–13
Takeda A, Tamano H (2020) New insight into Parkinson’s disease pathogenesis from reactive oxygen species-mediated extracellular Zn2+ influx. J Trace Elem Med Biol 61:126545
Tamano H, Sato Y, Takiguchi M, Murakami T, Fukuda T, Kawagishi H, Suzuki M, Takeda A (2019) CA1 LTP attenuated by corticosterone is canceled by effusol via rescuing intracellular Zn2+ dysregulation. Cell Mol Neurobiol 39:975–983
Funding
The authors received no funding in the present paper. The present paper contains non-financial interests.
Author information
Authors and Affiliations
Contributions
Conceptualization, Atsushi Takeda. Data curation, Haruna Tamano, Mako Takiguchi, Nana Saeki. Formal analysis, Haruna Tamano, Mako Takiguchi. Investigation, Mako Takiguchi, Nana Saeki, Misa Katahira, Aoi Shioya, Yukino Tanaka, Mako Egawa, Toshiyuki Fukuda. Methodology, Atsushi Takeda. Project administration, Atsushi Takeda. Resources, Atsushi Takeda. Software, Hiroki Ikeda. Supervision, Haruna Tamano. Validation, Haruna Tamano. Roles/writing—original draft, Haruna Tamano. Writing—review & editing, Atsushi Takeda.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The Ethics Committee for Experimental Animals has permitted the present study in the University of Shizuoka. There is no informed consent because the present study does not deal with human study. The present paper has been approved by all named authors.
Consent for Publication
The present paper, which is original, has not been published before and is not currently being considered for publication elsewhere.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamano, H., Takiguchi, M., Saeki, N. et al. Dehydroeffusol Pprevents Amyloid β1-42-mediated Hippocampal Neurodegeneration via Reducing Intracellular Zn2+ Toxicity. Mol Neurobiol 58, 3603–3613 (2021). https://doi.org/10.1007/s12035-021-02364-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02364-3