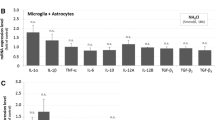
Abstract
Astrocytes are functionally diverse glial cells that maintain blood-brain barrier (BBB) integrity, provide metabolic and trophic support, and react to pathogens or harmful stimuli through inflammatory response. Impairment of astrocyte functions has been implicated in hepatic encephalopathy (HE), a neurological complication associated with hyperammonemia. Although hyperammonemia is more common in adults, ammonia gliotoxicity has been mainly studied in cultured astrocytes derived from neonate animals. However, these cells can sense and respond to stimuli in different ways from astrocytes obtained from adult animals. Thus, the aim of this study was to investigate the direct effects of ammonia on astrocyte cultures obtained from adult rats compared with those obtained from neonate rats. Our main findings pointed that ammonia increased the gene expression of proteins associated with BBB permeability, in addition to cause an inflammatory response and decrease the release of trophic factors, which were dependent on p38 mitogen-activated protein kinase (p38 MAPK)/nuclear factor κB (NFκB) pathways and aquaporin 4, in both neonatal and mature astrocytes. Considering the age, mature astrocytes presented an overall increase of the expression of inflammatory signaling components and a decrease of the expression of cytoprotective pathways, compared with neonatal astrocytes. Importantly, ammonia exposure in mature astrocytes potentiated the expression of the senescence marker p21, inflammatory response, activation of p38 MAPK/NFκB pathways, and the decrease of cytoprotective pathways. In this regard, ammonia can trigger and/or accelerate the inflammaging of mature astrocytes, a phenomenon characterized by an age-related chronic and low-grade inflammation, which may be implicated in HE neurological symptoms.





Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dallérac G, Zapata J, Rouach N (2018) Versatile control of synaptic circuits by astrocytes: where, when and how? Nat Rev Neurosci 19:729–743. https://doi.org/10.1038/s41583-018-0080-6
Gonçalves C-A, Rodrigues L, Bobermin LD, Zanotto C, Vizuete A, Quincozes-Santos A, Souza DO, Leite MC (2018) Glycolysis-derived compounds from astrocytes that modulate synaptic communication. Front Neurosci 12:1035. https://doi.org/10.3389/fnins.2018.01035
Michinaga S, Koyama Y (2019) Dual roles of astrocyte-derived factors in regulation of blood-brain barrier function after brain damage Int J Mol Sci 20:. https://doi.org/10.3390/ijms20030571,
Markiewicz I, Lukomska B (2006) The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp (Wars) 66:343–358
Colombo E, Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37:608–620. https://doi.org/10.1016/j.it.2016.06.006
Cunningham C, Dunne A, Lopez-Rodriguez AB (2018) Astrocytes: heterogeneous and dynamic phenotypes in neurodegeneration and innate immunity. Neuroscientist 1073858418809941. https://doi.org/10.1177/1073858418809941
Okun E, Griffioen KJ, Mattson MP (2011) Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 34:269–281. https://doi.org/10.1016/j.tins.2011.02.005
Bobermin LD, Roppa RHA, Quincozes-Santos A (2019) Adenosine receptors as a new target for resveratrol-mediated glioprotection. Biochim Biophys Acta Mol basis Dis 1865:634–647. https://doi.org/10.1016/j.bbadis.2019.01.004
Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59:242–255. https://doi.org/10.1002/glia.21094
Yi W, Schlüter D, Wang X (2019) Astrocytes in multiple sclerosis and experimental autoimmune encephalomyelitis: star-shaped cells illuminating the darkness of CNS autoimmunity. Brain Behav Immun 80:10–24. https://doi.org/10.1016/j.bbi.2019.05.029
Dasarathy S, Mookerjee RP, Rackayova V, Rangroo Thrane V, Vairappan B, Ott P, Rose CF (2017) Ammonia toxicity: from head to toe? Metab Brain Dis 32:529–538. https://doi.org/10.1007/s11011-016-9938-3
Walker V (2014) Ammonia metabolism and hyperammonemic disorders. In: Advances in Clinical Chemistry. Elsevier, pp 73–150
Felipo V (2013) Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14:851–858. https://doi.org/10.1038/nrn3587
Jayakumar AR, Norenberg MD (2016) Glutamine synthetase: role in neurological disorders. Adv Neurobiol 13:327–350. https://doi.org/10.1007/978-3-319-45096-4_13
Bobermin LD, Souza DO, Gonçalves C-A, Quincozes-Santos A (2018) Resveratrol prevents ammonia-induced mitochondrial dysfunction and cellular redox imbalance in C6 astroglial cells. Nutr Neurosci 21:276–285. https://doi.org/10.1080/1028415X.2017.1284375
Bobermin LD, Quincozes-Santos A, Guerra MC, Leite MC, Souza DO, Gonçalves CA, Gottfried C (2012) Resveratrol prevents ammonia toxicity in astroglial cells. PLoS One 7:e52164. https://doi.org/10.1371/journal.pone.0052164
Hernández-Rabaza V, Cabrera-Pastor A, Taoro-González L, Malaguarnera M, Agustí A, Llansola M, Felipo V (2016) Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane. J Neuroinflammation 13:41. https://doi.org/10.1186/s12974-016-0505-y
Oja SS, Saransaari P, Korpi ER (2017) Neurotoxicity of ammonia. Neurochem Res 42:713–720. https://doi.org/10.1007/s11064-016-2014-x
Rama Rao KV, Jayakumar AR, Tong X, Curtis KM, Norenberg MD (2010) Brain aquaporin-4 in experimental acute liver failure. J Neuropathol Exp Neurol 69:869–879. https://doi.org/10.1097/NEN.0b013e3181ebe581
Wright G, Soper R, Brooks HF, Stadlbauer V, Vairappan B, Davies NA, Andreola F, Hodges S et al (2010) Role of aquaporin-4 in the development of brain oedema in liver failure. J Hepatol 53:91–97. https://doi.org/10.1016/j.jhep.2010.02.020
Görg B, Karababa A, Shafigullina A, Bidmon HJ, Häussinger D (2015) Ammonia-induced senescence in cultured rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Glia 63:37–50. https://doi.org/10.1002/glia.22731
Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD (2012) Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res 37:2569–2588. https://doi.org/10.1007/s11064-012-0868-0
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2017) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54:2969–2985. https://doi.org/10.1007/s12035-016-9880-8
Longoni A, Bellaver B, Bobermin LD, Santos CL, Nonose Y, Kolling J, dos Santos TM, de Assis AM et al (2018) Homocysteine induces glial reactivity in adult rat astrocyte cultures. Mol Neurobiol 55:1966–1976. https://doi.org/10.1007/s12035-017-0463-0
Santos CL, Bobermin LD, Souza DO, Quincozes-Santos A (2018) Leptin stimulates the release of pro-inflammatory cytokines in hypothalamic astrocyte cultures from adult and aged rats. Metab Brain Dis 33:2059–2063. https://doi.org/10.1007/s11011-018-0311-6
Santos CL, Roppa PHA, Truccolo P, Fontella FU, Souza DO, Bobermin LD, Quincozes-Santos A (2018) Age-dependent neurochemical remodeling of hypothalamic astrocytes. Mol Neurobiol 55:5565–5579. https://doi.org/10.1007/s12035-017-0786-x
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS One 8:e60282. https://doi.org/10.1371/journal.pone.0060282
Bellaver B, Dos Santos JP, Leffa DT et al (2018) Systemic inflammation as a driver of brain injury: the astrocyte as an emerging player. Mol Neurobiol 55:2685–2695. https://doi.org/10.1007/s12035-017-0526-2
Christensen LB, Woods TA, Carmody AB, Caughey B, Peterson KE (2014) Age-related differences in neuroinflammatory responses associated with a distinct profile of regulatory markers on neonatal microglia. J Neuroinflammation 11:70. https://doi.org/10.1186/1742-2094-11-70
Gárate I, Garcia-Bueno B, Madrigal JLM, Caso JR, Alou L, Gomez-Lus ML, Micó JA, Leza JC (2013) Stress-induced neuroinflammation: role of the toll-like receptor-4 pathway. Biol Psychiatry 73:32–43. https://doi.org/10.1016/j.biopsych.2012.07.005
Klein RS, Hunter CA (2017) Protective and pathological immunity during CNS infections. Immunity 46:891–909. https://doi.org/10.1016/j.immuni.2017.06.012
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S4–S9. https://doi.org/10.1093/gerona/glu057
Griñan-Ferré C, Palomera-Ávalos V, Puigoriol-Illamola D, Camins A, Porquet D, Plá V, Aguado F, Pallàs M (2016) Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp Gerontol 80:57–69. https://doi.org/10.1016/j.exger.2016.03.014
Pizza V, Agresta A, D’Acunto CW et al (2011) Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS Neurol Disord Drug Targets 10:621–634
Bobermin LD, Arús BA, Leite MC, Souza DO, Gonçalves CA, Quincozes-Santos A (2016) Gap junction intercellular communication mediates ammonia-induced neurotoxicity. Neurotox Res 29:314–324. https://doi.org/10.1007/s12640-015-9581-5
Bobermin LD, Hansel G, Scherer EBS, Wyse ATS, Souza DO, Quincozes-Santos A, Gonçalves CA (2015) Ammonia impairs glutamatergic communication in astroglial cells: protective role of resveratrol. Toxicol in Vitro 29:2022–2029. https://doi.org/10.1016/j.tiv.2015.08.008
Wartchow KM, Tramontina AC, de Souza DF, Biasibetti R, Bobermin LD, Gonçalves CA (2016) Insulin stimulates S100B secretion and these proteins antagonistically modulate brain glucose metabolism. Neurochem Res 41:1420–1429. https://doi.org/10.1007/s11064-016-1851-y
Zanotto C, Abib RT, Batassini C, Tortorelli LS, Biasibetti R, Rodrigues L, Nardin P, Hansen F et al (2013) Non-specific inhibitors of aquaporin-4 stimulate S100B secretion in acute hippocampal slices of rats. Brain Res 1491:14–22. https://doi.org/10.1016/j.brainres.2012.10.065
Rose C, Kresse W, Kettenmann H (2005) Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J Biol Chem 280:20937–20944. https://doi.org/10.1074/jbc.M412448200
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Bodega G, Suárez I, López-Fernández LA, García MI, Köber M, Penedo M, Luna M, Juárez S et al (2012) Ammonia induces aquaporin-4 rearrangement in the plasma membrane of cultured astrocytes. Neurochem Int 61:1314–1324. https://doi.org/10.1016/j.neuint.2012.09.008
Bender AS, Norenberg MD (1996) Effects of ammonia on L-glutamate uptake in cultured astrocytes. Neurochem Res 21:567–573. https://doi.org/10.1007/bf02527755
Gregorios JB, Mozes LW, Norenberg MD (1985) Morphologic effects of ammonia on primary astrocyte cultures II Electron microscopic studies. J Neuropathol Exp Neurol 44:404–414. https://doi.org/10.1097/00005072-198507000-00004
Murthy CR, Rama Rao KV, Bai G, Norenberg MD (2001) Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res 66:282–288. https://doi.org/10.1002/jnr.1222
Neary JT, Woodson C, Blicharska J, Norenberg LOB, Norenberg MD (1990) Effect of ammonia on calcium homeostasis in primary astrocyte cultures. Brain Res 524:231–235. https://doi.org/10.1016/0006-8993(90)90696-9
Neary JT, Norenberg LO, Gutierrez MP, Norenberg MD (1987) Hyperammonemia causes altered protein phosphorylation in astrocytes. Brain Res 437:161–164. https://doi.org/10.1016/0006-8993(87)91538-1
Suárez I, Bodega G, Fernández B (2002) Glutamine synthetase in brain: effect of ammonia. Neurochem Int 41:123–142. https://doi.org/10.1016/S0197-0186(02)00033-5
Li Y, Wang S, Ran K et al (2015) Differential hippocampal protein expression between normal aged rats and aged rats with postoperative cognitive dysfunction: a proteomic analysis. Mol Med Rep 12:2953–2960. https://doi.org/10.3892/mmr.2015.3697
Shen Y, Gao H, Shi X, Wang N, Ai D, Li J, Ouyang L, Yang J et al (2014) Glutamine synthetase plays a role in d-galactose-induced astrocyte aging in vitro and in vivo. Exp Gerontol 58:166–173. https://doi.org/10.1016/j.exger.2014.08.006
Souza DG, Bellaver B, Raupp GS, Souza DO, Quincozes-Santos A (2015) Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int 90:93–97. https://doi.org/10.1016/j.neuint.2015.07.016
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53. https://doi.org/10.1038/nrn1824
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019) Blood-brain barrier: from physiology to disease and back. Physiol Rev 99:21–78. https://doi.org/10.1152/physrev.00050.2017
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14:265–277. https://doi.org/10.1038/nrn3468
Potokar M, Jorgačevski J, Zorec R (2016) Astrocyte aquaporin dynamics in health and disease. Int J Mol Sci 17. https://doi.org/10.3390/ijms17071121
Pan C-F, Zhu S-M, Zheng Y-Y (2010) Ammonia induces upregulation of aquaporin-4 in neocortical astrocytes of rats through the p38 mitogen-activated protein kinase pathway. Chin Med J 123:1888–1892
Rama Rao KV, Chen M, Simard JM, Norenberg MD (2003) Increased aquaporin-4 expression in ammonia-treated cultured astrocytes. Neuroreport 14:2379–2382. https://doi.org/10.1097/00001756-200312190-00018
Rama Rao KV, Norenberg MD (2007) Aquaporin-4 in hepatic encephalopathy. Metab Brain Dis 22:265–275. https://doi.org/10.1007/s11011-007-9063-4
He L, Zhang X, Wei X, Li Y (2014) Progesterone attenuates aquaporin-4 expression in an astrocyte model of ischemia/reperfusion. Neurochem Res 39:2251–2261. https://doi.org/10.1007/s11064-014-1427-7
Katoozi S, Skauli N, Zahl S, Deshpande T, Ezan P, Palazzo C, Steinhäuser C, Frigeri A et al (2020) Uncoupling of the astrocyte syncytium differentially affects AQP4 isoforms. Cells 9:382. https://doi.org/10.3390/cells9020382
Skowrońska K, Obara-Michlewska M, Czarnecka A, Dąbrowska K, Zielińska M, Albrecht J (2019) Persistent overexposure to N-methyl-d-aspartate (NMDA) calcium-dependently downregulates glutamine synthetase, aquaporin 4, and Kir4.1 channel in mouse cortical astrocytes. Neurotox Res 35:271–280. https://doi.org/10.1007/s12640-018-9958-3
Vandebroek A, Yasui M (2020) Regulation of AQP4 in the central nervous system. IJMS 21:1603. https://doi.org/10.3390/ijms21051603
Fukuda AM, Badaut J (2012) Aquaporin 4: a player in cerebral edema and neuroinflammation. J Neuroinflammation 9:279. https://doi.org/10.1186/1742-2094-9-279
Ikeshima-Kataoka H (2016) Neuroimmunological implications of AQP4 in astrocytes. Int J Mol Sci 17:1306. https://doi.org/10.3390/ijms17081306
Akdemir G, Ratelade J, Asavapanumas N, Verkman AS (2014) Neuroprotective effect of aquaporin-4 deficiency in a mouse model of severe global cerebral ischemia produced by transient 4-vessel occlusion. Neurosci Lett 574:70–75. https://doi.org/10.1016/j.neulet.2014.03.073
Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS (2011) Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J 25:1556–1566. https://doi.org/10.1096/fj.10-177279
Liang F, Luo C, Xu G, Su F, He X, Long S, Ren H, Liu Y et al (2015) Deletion of aquaporin-4 is neuroprotective during the acute stage of micro traumatic brain injury in mice. Neurosci Lett 598:29–35. https://doi.org/10.1016/j.neulet.2015.05.006
Rama Rao KV, Verkman AS, Curtis KM, Norenberg MD (2014) Aquaporin-4 deletion in mice reduces encephalopathy and brain edema in experimental acute liver failure. Neurobiol Dis 63:222–228. https://doi.org/10.1016/j.nbd.2013.11.018
Verkman AS, Smith AJ, Phuan P-W, Tradtrantip L, Anderson MO (2017) The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin Ther Targets 21:1161–1170. https://doi.org/10.1080/14728222.2017.1398236
Jayakumar AR, Rama Rao KV, Norenberg MD (2015) Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol 5:S21–S28. https://doi.org/10.1016/j.jceh.2014.07.006
Rodrigo R, Cauli O, Gomez-Pinedo U et al (2010) Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139:675–684. https://doi.org/10.1053/j.gastro.2010.03.040
Santos CL, Bobermin LD, Souza DG, Bellaver B, Bellaver G, Arús BA, Souza DO, Gonçalves CA et al (2015) Lipoic acid and N-acetylcysteine prevent ammonia-induced inflammatory response in C6 astroglial cells: The putative role of ERK and HO1 signaling pathways. Toxicol in Vitro 29:1350–1357. https://doi.org/10.1016/j.tiv.2015.05.023
Dresselhaus EC, Meffert MK (2019) Cellular specificity of NF-κB function in the nervous system. Front Immunol 10:1043. https://doi.org/10.3389/fimmu.2019.01043
Wang X, Yang L, Yang L, Xing F, Yang H, Qin L, Lan Y, Wu H et al (2017) Gypenoside IX suppresses p38 MAPK/Akt/NFκB signaling pathway activation and inflammatory responses in astrocytes stimulated by proinflammatory mediators. Inflammation 40:2137–2150. https://doi.org/10.1007/s10753-017-0654-x
Nito C, Kamada H, Endo H, Narasimhan P, Lee YS, Chan PH (2012) Involvement of mitogen-activated protein kinase pathways in expression of the water channel protein aquaporin-4 after ischemia in rat cortical astrocytes. J Neurotrauma 29:2404–2412. https://doi.org/10.1089/neu.2012.2430
Miller AH, Haroon E, Raison CL, Felger JC (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297–306. https://doi.org/10.1002/da.22084
De Luca C, Colangelo AM, Alberghina L, Papa M (2018) Neuro-immune hemostasis: homeostasis and diseases in the central nervous system. Front Cell Neurosci 12:459. https://doi.org/10.3389/fncel.2018.00459
Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ et al (2012) Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122:2454–2468. https://doi.org/10.1172/JCI60842
Butterworth RF (2013) The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol 10:522–528. https://doi.org/10.1038/nrgastro.2013.99
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol in Vitro 28:479–484. https://doi.org/10.1016/j.tiv.2014.01.006
Souza DG, Bellaver B, Bobermin LD, Souza DO, Quincozes-Santos A (2016) Anti-aging effects of guanosine in glial cells. Purinergic Signal 12:697–706. https://doi.org/10.1007/s11302-016-9533-4
Patterson SL (2015) Immune dysregulation and cognitive vulnerability in the aging brain: interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology 96:11–18. https://doi.org/10.1016/j.neuropharm.2014.12.020
Spulber S, Schultzberg M (2010) Connection between inflammatory processes and transmittor function-modulatory effects of interleukin-1. Prog Neurobiol 90:256–262. https://doi.org/10.1016/j.pneurobio.2009.10.015
Takao T, Nagano I, Tojo C, Takemura T, Makino S, Hashimoto K, de Souza EB (1996) Age-related reciprocal modulation of interleukin-1βand interleukin-1 receptors in the mouse brain-endocrine-immune axis. Neuroimmunomodulation 3:205–212. https://doi.org/10.1159/000097272
Agusti A, Cauli O, Rodrigo R, Llansola M, Hernandez-Rabaza V, Felipo V (2011) p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts. Gut 60:1572–1579. https://doi.org/10.1136/gut.2010.236083
Bachstetter AD, Van Eldik LJ (2010) The p38 MAP kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis 1:199–211
Rosciszewski G, Cadena V, Murta V, Lukin J, Villarreal A, Roger T, Ramos AJ (2018) Toll-like receptor 4 (TLR4) and triggering receptor expressed on myeloid cells-2 (TREM-2) activation balance astrocyte polarization into a proinflammatory phenotype. Mol Neurobiol 55:3875–3888. https://doi.org/10.1007/s12035-017-0618-z
Wilhelm I, Nyúl-Tóth Á, Kozma M, Farkas AE, Krizbai IA (2017) Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol 313:H1000–H1012. https://doi.org/10.1152/ajpheart.00106.2017
Hanke ML, Kielian T (2011) Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci 121:367–387. https://doi.org/10.1042/CS20110164
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801. https://doi.org/10.1016/j.cell.2006.02.015
Gárate I, García-Bueno B, Madrigal JLM, Bravo L, Berrocoso E, Caso JR, Micó JA, Leza JC (2011) Origin and consequences of brain toll-like receptor 4 pathway stimulation in an experimental model of depression. J Neuroinflammation 8:151. https://doi.org/10.1186/1742-2094-8-151
Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K (2007) Innate immune receptor expression in normal brain aging. Neuroscience 146:248–254. https://doi.org/10.1016/j.neuroscience.2007.01.004
Kong H, Zeng X-N, Fan Y, Yuan ST, Ge S, Xie WP, Wang H, Hu G (2014) Aquaporin-4 knockout exacerbates corticosterone-induced depression by inhibiting astrocyte function and hippocampal neurogenesis. CNS Neurosci Ther 20:391–402. https://doi.org/10.1111/cns.12222
Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL et al (2011) Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci 31:6392–6397. https://doi.org/10.1523/JNEUROSCI.6249-10.2011
Galland F, Negri E, Da Ré C et al (2017) Hyperammonemia compromises glutamate metabolism and reduces BDNF in the rat hippocampus. Neurotoxicology 62:46–55. https://doi.org/10.1016/j.neuro.2017.05.006
Dhanda S, Gupta S, Halder A, Sunkaria A, Sandhir R (2018) Systemic inflammation without gliosis mediates cognitive deficits through impaired BDNF expression in bile duct ligation model of hepatic encephalopathy. Brain Behav Immun 70:214–232. https://doi.org/10.1016/j.bbi.2018.03.002
Ahmad AS, Zhuang H, Doré S (2006) Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience 141:1703–1708. https://doi.org/10.1016/j.neuroscience.2006.05.035
Chen-Roetling J, Kamalapathy P, Cao Y, Song W, Schipper HM, Regan RF (2017) Astrocyte heme oxygenase-1 reduces mortality and improves outcome after collagenase-induced intracerebral hemorrhage. Neurobiol Dis 102:140–146. https://doi.org/10.1016/j.nbd.2017.03.008
Yu X, Song N, Guo X, Jiang H, Zhang H, Xie J (2016) Differences in vulnerability of neurons and astrocytes to heme oxygenase-1 modulation: implications for mitochondrial ferritin. Sci Rep 6:24200. https://doi.org/10.1038/srep24200
Kim Y, Park J, Choi YK (2019) The role of astrocytes in the central nervous system focused on BK channel and heme oxygenase metabolites: a review. Antioxidants 8:121. https://doi.org/10.3390/antiox8050121
Wegiel B, Nemeth Z, Correa-Costa M, Bulmer AC, Otterbein LE (2014) Heme oxygenase-1: a metabolic nike. Antioxid Redox Signal 20:1709–1722. https://doi.org/10.1089/ars.2013.5667
Görg B, Bidmon H-J, Häussinger D (2013) Gene expression profiling in the cerebral cortex of patients with cirrhosis with and without hepatic encephalopathy. Hepatology 57:2436–2447. https://doi.org/10.1002/hep.26265
Oenarto J, Karababa A, Castoldi M, Bidmon HJ, Görg B, Häussinger D (2016) Ammonia-induced miRNA expression changes in cultured rat astrocytes. Sci Rep 6:18493. https://doi.org/10.1038/srep18493
Warskulat U, Görg B, Bidmon H-J, Müller HW, Schliess F, Häussinger D (2002) Ammonia-induced heme oxygenase-1 expression in cultured rat astrocytes and rat brain in vivo. Glia 40:324–336. https://doi.org/10.1002/glia.10128
Görg B, Karababa A, Häussinger D (2018) Hepatic encephalopathy and astrocyte senescence. J Clin Exp Hepatol 8:294–300. https://doi.org/10.1016/j.jceh.2018.05.003
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24:2463–2479. https://doi.org/10.1101/gad.1971610
Nagelhus EA, Amiry-Moghaddam M, Bergersen LH, Bjaalie JG, Eriksson J, Gundersen V, Leergaard TB, Morth JP et al (2013) The glia doctrine: addressing the role of glial cells in healthy brain ageing. Mech Ageing Dev 134:449–459. https://doi.org/10.1016/j.mad.2013.10.001
Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H (2011) Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34:3–11. https://doi.org/10.1111/j.1460-9568.2011.07738.x
Chen QM (2000) Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann N Y Acad Sci 908:111–125. https://doi.org/10.1111/j.1749-6632.2000.tb06640.x
Umapathy S, Dhiman RK, Grover S, Duseja A, Chawla YK (2014) Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Am J Gastroenterol 109:1011–1019. https://doi.org/10.1038/ajg.2014.107
Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, Hafeezullah M et al (2010) Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 138:2332–2340. https://doi.org/10.1053/j.gastro.2010.02.015
Braissant O, McLin VA, Cudalbu C (2013) Ammonia toxicity to the brain. J Inherit Metab Dis 36:595–612. https://doi.org/10.1007/s10545-012-9546-2
Code Availability
Not applicable.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Universidade Federal do Rio Grande do Sul, and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal experiments were performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and Brazilian Society for Neuroscience and Behavior recommendations for animal care. The experimental protocols were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number 21215).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bobermin, L.D., Roppa, R.H.A., Gonçalves, CA. et al. Ammonia-Induced Glial-Inflammaging. Mol Neurobiol 57, 3552–3567 (2020). https://doi.org/10.1007/s12035-020-01985-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01985-4