Abstract
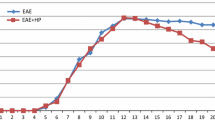
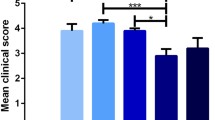
Chronic relapsing experimental allergic encephalomyelitis (CR-EAE) exhibits neuropathological and immunological dysfunctions similar to those found in multiple sclerosis (MS) and has been used as an animal model of MS. Inflammatory infiltrates and oxidative stress have been linked to the development of both diseases. Ethanolamine plasmalogen derivates have been shown to be powerful antioxidants and immunomodulators. Therefore, the objective of this study was to analyse inflammatory infiltrates, the state of the oxidative defences and the possible protective effects of calcium, magnesium and phosphate ethanolamine (EAP) in the CR-EAE rat hippocampus. To this aim, we evaluated, by immunohistochemistry, T cell infiltrates, Iba-1+ (a marker of activated microglia) immunoreactivity and TUNEL (+) cells. We also measured the protein levels and activity of the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GP) and glutathione reductase (GR). In addition, reduced (GSH) and oxidized (GSSG) glutathione levels, lipid peroxidation and cholesterol as well as desmosterol content were determined. We found an increase in T cell infiltrates and Iba1+ immunoreactivity, lipid peroxidation, SOD, GP and GR activities as well as enhanced cholesterol levels and a decrease in CAT activity, GSH and desmosterol levels in the first and second attack in the CR-EAE rat hippocampus. Pretreatment of CR-EAE rats with EAP led to a delay in the onset of the clinical signs of the disease as well as a decrease in inflammatory infiltrates and alterations of the antioxidant defences in the hippocampus. Altogether, the present results suggest a protective role of EAP in the CR-EAE rat hippocampus.










Similar content being viewed by others
References
Polman CH, Uitdehaag BM (2000) Drug treatment of multiple sclerosis. BMJ 321:490–494
Conlon P, Oksenberg JR, Zhang J, Steinman L (1999) The immunobiology of multiple sclerosis: an autoimmune disease of the central nervous system. Neurobiol Dis 6:149–166
Gold R, Hartung HP, Toyka KV (2000) Animal models for autoimmune demyelinating disorders of the nervous system. Mol Med Today 6:88–91
Slavin A, Ewing C, Liu J, Ichikawa M, Slavin J, Bernard CC (1998) Induction of a multiple sclerosis-like disease in mice with an immunodominant epitope of myelin oligodendrocyte glycoprotein. Autoimmunity 28:109–120
Steinman L (2001) Multiple sclerosis: a two-stage disease. Nat Immunol 2:762–764
Sobel RA, Blanchette BW, Bhan AK, Colvin RB (1984) The immunopathology of experimental allergic encephalomyelitis. I. Quantitative analysis of inflammatory cells in situ. J Immunol 132:2393–2401
Polman CH, Dijkstra CD, Sminia T, Koetsier JC (1986) Immunohistological analysis of macrophages in the central nervous system of Lewis rats with acute experimental allergic encephalomyelitis. J Neuroimmunol 11:215–222
Cross AH, Raine CS (1991) Central nervous system endothelial cell-polymorphonuclear cell interactions during autoimmune demyelination. Am J Pathol 139:1401–1409
Babior BM (1984) Oxidants from phagocytes: agents of defense and destruction. Blood 64:959–966
Banati RB, Gehrmann J, Schubert P, Kreutzberg GW (1993) Cytotoxicity of microglia. Glia 7:111–118
Colton CA, Gilbert DL (1987) Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett 223:284–288
Matsumoto Y, Hara N, Tanaka R, Fujiwara M (1986) Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol 136:3668–3676
Matsumoto Y, Ohmori K, Fujiwara M (1992) Microglial and astroglial reactions to inflammatory lesions of experimental autoimmune encephalomyelitis in the rat central nervous system. J Neuroimmunol 37:23–33
Vass K, Lassmann H, Wekerle H, Wisniewski HM (1986) The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol 70:149–160
Fialkow L, Wang Y, Downey GP (2007) Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 42:153–164
Gilgun-Sherki Y, Melamed E, Offen D (2004) The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 251:261–268
Calabrese M, Favaretto A, Martini V, Gallo P (2012) Grey matter lesions in MS: From histology to clinical implications. Prion 7:20–27
Bobholz JA, Rao SM (2003) Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol 16:283–288
Rao SM, Leo GJ, Bernardin L, Unverzagt F (1991) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41:685–691
Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY (2008) Regional hippocampal atrophy in multiple sclerosis. Brain 131:1134–1141
Sajad M, Zargan J, Chawla R, Umar S, Sadaqat M, Khan HA (2009) Hippocampal neurodegeneration in experimental autoimmune encephalomyelitis (EAE): potential role of inflammation activated myeloperoxidase. Mol Cell Biochem 328:183–188
Mann EO, Greenfield SA (2003) Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol 551:539–550
Aguado-Llera D, Puebla-Jimenez L, Yebenes-Gregorio L, Arilla-Ferreiro E (2007) Alteration of the somatostatinergic system in the striatum of rats with acute experimental autoimmune encephalomyelitis. Neuroscience 148:238–249
Nisticò R, Mango D, Mandolesi G, Piccinin S, Berretta N, Pignatelli M, Feligioni M, Musella A et al (2013) Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PLoS One 8:e54666
Chaudhuri A, Behan PO (2003) Natalizumab for relapsing multiple sclerosis. N Engl J Med 348:1598–1599
Doggrell SA (2003) Is natalizumab a breakthrough in the treatment of multiple sclerosis? Expert Opin Pharmacother 4:999–1001
Miller DM, Rudick RA, Baier M, Cutter G, Doughtery DS, Weinstock-Guttman B, Mass MK, Fisher E et al (2003) Factors that predict health-related quality of life in patients with relapsing-remitting multiple sclerosis. Mult Scler 9:1–5
Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E et al (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277:21453–21457
Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, Li XK (2003) Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther 305:70–77
Mazda ME, Brosch JR, Wiens AL, Bonnin J, Kamer AP, Mattson DH, Snook RJ (2013) A case of natalizumab-associated progressive multifocal leukoencephalopathy with repeated negative CSF JC virus testing. Int J Neurosci 123:353–357
Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD (2005) Macrophages and neurodegeneration. Brain Res Brain Res Rev 48:185–195
van Meeteren ME, Teunissen CE, Dijkstra CD, van Tol EA (2005) Antioxidants and polyunsaturated fatty acids in multiple sclerosis. Eur J Clin Nutr 59:1347–1361
Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P (2008) Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatry 79:1224–1229
Andriamampandry C, Freysz L, Kanfer JN, Dreyfus H, Massarelli R (1989) Conversion of ethanolamine, monomethylethanolamine and dimethylethanolamine to choline-containing compounds by neurons in culture and by the rat brain. Biochem J 264:555–562
Buratta S, Hamberger A, Ryberg H, Nystrom B, Sandberg M, Mozzi R (1998) Effect of serine and ethanolamine administration on phospholipid-related compounds and neurotransmitter amino acids in the rabbit hippocampus. J Neurochem 71:2145–2150
Marshall DL, De Micheli E, Bogdanov MB, Wurtman RJ (1996) Effects of ethanolamine (Etn) administration on Etn and choline (Ch) levels in plasma, brain extracellular fluid (ECF) and brain tissue, and on brain phospholipid levels in rats: an in vivo study. Neurosci Res Commun 18:87–96
Wilson R, Tocher DR (1991) Lipid and fatty acid composition is altered in plaque tissue from multiple sclerosis brain compared with normal brain white matter. Lipids 26:9–15
Kuczynski B, Reo NV (2006) Evidence that plasmalogen is protective against oxidative stress in the rat brain. Neurochem Res 31:639–656
Maeba R, Ueta N (2004) A novel antioxidant action of ethanolamine plasmalogens in lowering the oxidizability of membranes. Biochem Soc Trans 32:141–143
Jagannatha HM, Sastry PS (1981) Ethanolamine plasmalogen & cholesterol ester metabolism in experimental allergic encephalomyelitis. Indian J Biochem Biophys 18:411–416
Guo L, Davies SS (2013) Bioactive aldehyde-modified phosphatidylethanolamines. Biochimie 95:74–78
Shiratsuchi A, Ichiki M, Okamoto Y, Ueda N, Sugimoto N, Takuwa Y, Nakanishi Y (2009) Inhibitory effect of N-palmitoylphosphatidylethanolamine on macrophage phagocytosis through inhibition of Rac1 and Cdc42. J Biochem 145:43–50
Feurer C, Prentice DE, Cammisuli S (1985) Chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. J Neuroimmunol 10:159–166
Massella A, D'Intino G, Fernández M, Sivilia S, Lorenzini L, Giatti S, Melcangi RC, Calzà L et al (2012) Gender effect on neurodegeneration and myelin markers in an animal model for multiple sclerosis. BMC Neurosci 13:12
Gold DP, Schroder K, Powell HC, Kelly CJ (1997) Nitric oxide and the immunomodulation of experimental allergic encephalomyelitis. Eur J Immunol 27:2863–2869
Glowinski J, Iversen L (1966) Regional studies of catecholamines in the rat brain. Subcellullar distribution of endogenous and exogenous catecholamines in various brain regions. Biochem Pharmacol 15:977–987
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ottolenghi CE (1959) Bol Trab Soc Cir B Aires 43:47–50
Minami M, Yoshikawa H (1979) A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta 92:337–342
McCord JM, Fridovich I (1969) The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem 244:6056–6063
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Mumby SM, Kahn RA, Manning DR, Gilman AG (1986) Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A 83:265–269
Penn RB, Pronin AN, Benovic JL (2000) Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med 10:81–89
Sánchez-Wandelmer J, Davalos A, Herrera E, Giera M, Cano S, de la Pena G, Lasuncion MA, Busto R (2009) Inhibition of cholesterol biosynthesis disrupts lipid raft/caveolae and affects insulin receptor activation in 3T3-L1 preadipocytes. Biochim Biophys Acta 1788:1731–1739
Abourbeh G, Thézé B, Maroy R, Dubois A, Brulon V, Fontyn Y, Dollé F, Tavitian B et al (2012) Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [18F]DPA-714. J Neurosci 32:5728–5736
Barnett MH, Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55:458–468
Constantinescu CS, Farooqi N, O'Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164:1079–1106
Hampton DW, Anderson J, Pryce G, Irvine KA, Giovannoni G, Fawcett JW, Compston A, Franklin RJ et al (2008) An experimental model of secondary progressive multiple sclerosis that shows regional variation in gliosis, remyelination, axonal and neuronal loss. J Neuroimmunol 201-202:200–211
Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R (2010) Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J Neuropathol Exp Neurol 69:1017–1033
Acharjee S, Nayani N, Tsutsui M, Hill MN, Ousman SS, Pittman QJ (2013) Altered cognitive-emotional behavior in early experimental autoimmune encephalitis--cytokine and hormonal correlates. Brain Behav Immun 33:164–172
Elong Ngono A, Pettré S, Salou M, Bahbouhi B, Soulillou JP, Brouard S, Laplaud DA (2012) Frequency of circulating autoreactive T cells committed to myelin determinants in relapsing-remitting multiple sclerosis patients. Clin Immunol 144:117–126
Martin R, Jaraquemada D, Flerlage M, Richert J, Whitaker J, Long EO, McFarlin DE, McFarland HF (1990) Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol 145:540–548
MacMicking JD, Willenborg DO, Weidemann MJ, Rockett KA, Cowden WB (1992) Elevated secretion of reactive nitrogen and oxygen intermediates by inflammatory leukocytes in hyperacute experimental autoimmune encephalomyelitis: enhancement by the soluble products of encephalitogenic T cells. J Exp Med 176:303–307
Scott GS, Bolton C (2000) L-arginine modifies free radical production and the development of experimental allergic encephalomyelitis. Inflamm Res 49:720–726
Scott GS, Williams KI, Bolton C (1996) A pharmacological study on the role of nitric oxide in the pathogenesis of experimental allergic encephalomyelitis. Inflamm Res 45:524–529
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Shimizu N, Kobayashi K, Hayashi K (1984) The reaction of superoxide radical with catalase. Mechanism of the inhibition of catalase by superoxide radical. J Biol Chem 259:4414–4418
Halliwell B, Gutteridge JM (1992) Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett 307:108–112
Liu XH, Kato H, Nakata N, Kogure K, Kato K (1993) An immunohistochemical study of copper/zinc superoxide dismutase and manganese superoxide dismutase in rat hippocampus after transient cerebral ischemia. Brain Res 625:29–37
Matsuyama T, Michishita H, Nakamura H, Tsuchiyama M, Shimizu S, Watanabe K, Sugita M (1993) Induction of copper-zinc superoxide dismutase in gerbil hippocampus after ischemia. J Cereb Blood Flow Metab 13:135–144
Tajouri L, Mellick AS, Ashton KJ, Tannenberg AE, Nagra RM, Tourtellotte WW, Griffiths LR (2003) Quantitative and qualitative changes in gene expression patterns characterize the activity of plaques in multiple sclerosis. Brain Res Mol Brain Res 119:170–183
Singh I, Paintlia AS, Khan M, Stanislaus R, Paintlia MK, Haq E, Singh AK, Contreras MA (2004) Impaired peroxisomal function in the central nervous system with inflammatory disease of experimental autoimmune encephalomyelitis animals and protection by lovastatin treatment. Brain Res 1022:1–11
Guy J, Qi X, Hauswirth WW (1998) Adeno-associated viral-mediated catalase expression suppresses optic neuritis in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A 95:13847–13852
Liu Y, Teige I, Birnir B, Issazadeh-Navikas S (2006) Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med 12:518–525
Ruuls SR, Bauer J, Sontrop K, Huitinga I, Hart BA, Dijkstra CD (1995) Reactive oxygen species are involved in the pathogenesis of experimental allergic encephalomyelitis in Lewis rats. J Neuroimmunol 56:207–217
Zargari M, Allameh A, Sanati MH, Tiraihi T, Lavasani S, Emadyan O (2007) Relationship between the clinical scoring and demyelination in central nervous system with total antioxidant capacity of plasma during experimental autoimmune encephalomyelitis development in mice. Neurosci Lett 412:24–28
Calabrese V, Scapagnini G, Ravagna A, Bella R, Butterfield DA, Calvani M, Pennisi G, Giuffrida Stella AM (2003) Disruption of thiol homeostasis and nitrosative stress in the cerebrospinal fluid of patients with active multiple sclerosis: evidence for a protective role of acetylcarnitine. Neurochem Res 28:1321–1328
Ilhan A, Akyol O, Gurel A, Armutcu F, Iraz M, Oztas E (2004) Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic Biol Med 37:386–394
Liu Y, Zhu B, Wang X, Luo L, Li P, Paty DW, Cynader MS (2003) Bilirubin as a potent antioxidant suppresses experimental autoimmune encephalomyelitis: implications for the role of oxidative stress in the development of multiple sclerosis. J Neuroimmunol 139:27–35
Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A 101:2070–2075
Kuehnle K, Crameri A, Kalin RE, Luciani P, Benvenuti S, Peri A, Ratti F, Rodolfo M et al (2008) Prosurvival effect of DHCR24/Seladin-1 in acute and chronic responses to oxidative stress. Mol Cell Biol 28:539–550
Gesquiere L, Loreau N, Minnich A, Davignon J, Blache D (1999) Oxidative stress leads to cholesterol accumulation in vascular smooth muscle cells. Free Radic Biol Med 27:134–145
Stein O, Oette K, Dabach Y, Hollander G, Ben Naim M, Stein Y (1992) Persistence of increased cholesteryl ester in human skin fibroblasts is caused by residual exogenous sphingomyelinase and is reversed by phospholipid liposomes. Biochim Biophys Acta 1165:153–159
Suzukawa M, Abbey M, Clifton P, Nestel PJ (1994) Effects of supplementing with vitamin E on the uptake of low density lipoprotein and the stimulation of cholesteryl ester formation in macrophages. Atherosclerosis 110:77–86
Zager RA, Kalhorn TF (2000) Changes in free and esterified cholesterol: hallmarks of acute renal tubular injury and acquired cytoresistance. Am J Pathol 157:1007–1016
Pitkanen AS, Halonen TO, Kilpelainen HO, Riekkinen PJ (1986) Cholesterol esterase activity in cerebrospinal fluid of multiple sclerosis patients. J Neurol Sci 74:45–53
Crameri A, Biondi E, Kuehnle K, Lutjohann D, Thelen KM, Perga S, Dotti CG, Nitsch RM et al (2006) The role of seladin-1/DHCR24 in cholesterol biosynthesis, APP processing and Abeta generation in vivo. EMBO J 25:432–443
Waterham HR, Koster J, Romeijn GJ, Hennekam RC, Vreken P, Andersson HC, FitzPatrick DR, Kelley RI et al (2001) Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am J Hum Genet 69:685–694
Wechsler A, Brafman A, Shafir M, Heverin M, Gottlieb H, Damari G, Gozlan-Kelner S, Spivak I et al (2003) Generation of viable cholesterol-free mice. Science 302:2087
Benvenuti S, Saccardi R, Luciani P, Urbani S, Deledda C, Cellai I, Francini F, Squecco R et al (2006) Neuronal differentiation of human mesenchymal stem cells: changes in the expression of the Alzheimer’s disease-related gene seladin-1. Exp Cell Res 312:2592–2604
Wu C, Miloslavskaya I, Demontis S, Maestro R, Galaktionov K (2004) Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432:640–645
Mangiardi M, Crawford D et al (2011) An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol 21:263–278
Crise B, Rose JK (1992) Human immunodeficiency virus type 1 glycoprotein precursor retains a CD4-p56lck complex in the endoplasmic reticulum. J Virol 66:2296–2301
Arcaro A, Grégoire C, Boucheron N, Stotz S, Palmer E, Malissen B, Luescher IF (2000) Essential role of CD8 palmitoylation in CD8 coreceptor function. J Immunol 165:2068–2076
Lee WT, Watson AR (2006) Single-cell analysis of lipid rafts in lymphocytes and in T cell-containing immunoconjugates. Curr Protoc Toxicol Chapter 2:Unit2.11
Legler DF, Doucey MA, Cerottini JC, Bron C, Luescher IF (2001) Selective inhibition of CTL activation by a dipalmitoyl-phospholipid that prevents the recruitment of signaling molecules to lipid rafts. FASEB J 15:1601–1603
Bosch B, Heipertz EL, Drake JR, Roche PA (2013) Major histocompatibility complex (MHC) class II-peptide complexes arrive at the plasma membrane in cholesterol-rich microclusters. JBC 288:13236–13242
Rimmerman N, Bradshaw HB, Kozela E, Levy R, Juknat A, Vogel Z (2012) Compartmentalization of endocannabinoids into lipid rafts in a microglial cell line devoid of caveolin-1. Br J Pharmacol 165:2436–2449
Hattersley KJ, Hein LK, Fuller M (2013) Lipid composition of membrane rafts, isolated with and without detergent, from the spleen of a mouse model of Gaucher disease. Biochem Biophys Res Commun 442:62–67
Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67:15–19
Aguado-Llera D, Puebla-Jimenez L, Barrios V, Hernandez-Pinto A, Arilla-Ferreiro E (2011) Role of ethanolamine phosphate in the hippocampus of rats with acute experimental autoimmune encephalomyelitis. Neurochem Int 58:22–34
Maeba R, Ueta N (2003) Ethanolamine plasmalogens prevent the oxidation of cholesterol by reducing the oxidizability of cholesterol in phospholipid bilayers. J Lipid Res 44:164–171
Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W (1999) Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem J 338:769–776
Acknowledgments
We wish to thank the staff of the Animal Center of the Universidad de Alcalá for their support with the animal care and handling. CIBEROBN is an initiative of the Instituto de Salud Carlos III (ISCIII), Spain. MOIR Mechanisms of Insulin Resistance.
Funding
This work was supported by the Ministerio de Ciencia e Innovación (SAF2010-22277), (SAF2011-29951) and (S2010/BMD-2423).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experiments performed in the present study conform to the guidelines set by the Animal Care Committee of Alcalá University as well as to the International guidelines on the ethical use of animals set by the Council of Europe (Protection of Animals Used for Experimentation, 1986 ETS No. 123), and all experimental protocols were previously approved.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Fig. 1
The image shows the cutting plane of the brain of control rats, rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE), rats with CR-EAE pre-treated with a calcium, magnesium and phosphate ethanolamine salt (EAP) and control rats pre-treated with EAP used to evaluate the infiltrates and activation of T cells, Iba1+ cells, IgG levels and TUNEL(+) cells in the hippocampus. This study has been carried out in similar areas of the medial section of the hippocampus rostro-caudal axis. (PPTX 5810 kb)
Supplementary Fig. 2
CD3+ and CD8+ cells. Immunohistochemical labelling of CD3+ and CD8+ cell infiltrates in the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) at three phases of the disease: first relapse, remission and second relapse, and effects of an EAP salt. The scale bar: 500 μm and 100 μm. The figure shows the most representative images of five independent stains of the hippocampal sagittal section of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) and effects of an EAP salt. (PPTX 650 kb)
Supplementary Fig. 3
Iba1+ cells. Panel A: Immunohistochemical labelling of Iba1+ cells in the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) at three phases of the disease: first relapse, remission and second relapse, and effects of an EAP salt. The scale bar: 100 μm. Panel B: Immunohistochemical labelling of Iba1+ cells and the score used to determine the degree of activation in the hippocampus. The scale bar: 50 μm. The figure shows the most representative images of five independent stains of the hippocampal sagittal section of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) and effects of an EAP salt. (PPTX 2012 kb)
Supplementary Fig. 4
Ig G levels. in the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) at three phases of the disease, and effects of EAP salt. Immunohistochemical labelling of IgG levels in the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) at three phases of the disease: first relapse, remission and second relapse, and effects of an EAP salt. The scale bar: 500 μm. The figure shows the most representative images of five independent stains of the sagittal section of the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) and effects of an EAP salt. (PPTX 455 kb)
Supplementary Fig. 5
TUNEL (+) cells. Immunohistochemical labelling of TUNEL (+) cells in the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) at three phases of the disease; first relapse, remission and second relapse, and effects of an EAP salt. The scale bar: 500 μm and 50 μm. The figure shows the most representative images of five independent stains of the sagittal section of the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) and effects of an EAP salt. (PPTX 546 kb)
Supplementary Fig. 6
The diagram depicts the alterations in the antioxidant defences and the possible mechanism of action of an ethanolamine phosphate salt (EAP) in the hippocampus of rats with chronic relapsing experimental allergic encephalomyelitis (CR-EAE) at the first attack. Panel A: In rats with CR-EAE, the increase of CD3+ cells, TNF-α and IFN-γ levels in the rat hippocampus enhances the amount and activation of resident Iba- 1+ cells. These cells begin to produce reactive oxygen species in the hippocampus. The alterations in the activity and quantity of enzymes involved in antioxidant defence also contribute to cell dysfunction. Our results showed an increase in the amount and activity of superoxide dismutase (SOD),a decrease in the amount and activity of catalase (CAT) and an impairment in the glutathione system (glutathione peroxidase (GP) and glutathione reductase (GR)). These alterations might contribute to the increase of lipid peroxidation and a dysfunction in the cellular activities of the hippocampus. Panel B: The CR-EAE + EAP rats show a decrease in the number of CD3+ cells in the hippocampus and less amount and activation of microglial cells, which involves a decrease in the number of oxidative radicals and lower levels of TNF-α. We have shown that the amount and activity of the antioxidant enzymes SOD, CAT, and the glutathione system (GR and GP) remain stable. All these findings could explain that in the CR-EAE + EAP rats, there is less lipid peroxidation and an improvement in the cellular activities of the hippocampus when compared with EAE-CR at the first attack. (PPTX 2123 kb)
Supplementary Fig. 7
The diagram depicts the alterations in the antioxidant defences and the possible mechanism of action of an ethanolamine phosphate salt (EAP) in the hippocampus of rats with CR-EAE at the second attack. Panel A: In the second relapse of CR-EAE, the amount of CD3+ cells and the amount and activity of Iba1+ cells are higher than in the first relapse. The increase in TNF-α levels in these rats could induce alterations in mitochondrial function that involve an increase of ROS and RNS. In addition, the alterations in the activity and amount of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and the impairment of the glutathione system (glutathione peroxidase (GP) and glutathione reductase (GR)) could contribute to the increase of lipid peroxidation, DNA damage and a dysfunction in the cellular activities of the hippocampus. Panel B: The EAP salt, in rats with CR-EAE, could reduce the alterations of cellular activities in the hippocampus by different mechanisms: these rats show a decrease in the number of CD3+ cells and less reduction in the amount and activation of microglial cells, which involves a decrease in the number of oxidative radicals and lower TNF-α levels. The present study also shows that the amount and activity of the antioxidant enzymes SOD, CAT, and the glutathione system (GR and GP) remain stable. (PPTX 2322 kb)
Rights and permissions
About this article
Cite this article
Perianes-Cachero, A., Lobo, M.V.T., Hernández-Pinto, A.M. et al. Oxidative Stress and Lymphocyte Alterations in Chronic Relapsing Experimental Allergic Encephalomyelitis in the Rat Hippocampus and Protective Effects of an Ethanolamine Phosphate Salt. Mol Neurobiol 57, 860–878 (2020). https://doi.org/10.1007/s12035-019-01774-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01774-8