Abstract
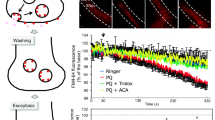
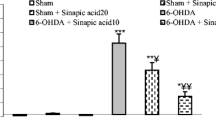
The herbicide paraquat (PQ) has been reported to enhance the risk of developing Parkinson’s disease (PD) from epidemiological studies. PQ-induced reactive oxygen species (ROS) are linked with a selective loss of nigrostriatal dopaminergic neurons. Here, we first report a unique mechanism of nigrostriatal dopaminergic degeneration, in which rapid intracellular Zn2+ dysregulation via PQ-induced ROS production causes PD in rats. When the substantia nigra pars compacta (SNpc) of rats was perfused with PQ, extracellular concentrations of glutamate and Zn2+ were increased and decreased, respectively, in the SNpc. These changes were ameliorated by co-perfusion with Trolox, an antioxidative agent. In in vitro slice experiments, PQ rapidly increased extracellular Zn2+ influx via AMPA receptor activation. Both loss of nigrostriatal dopaminergic neurons and increase in turning behavior in response to apomorphine were markedly reduced by coinjection of PQ and intracellular Zn2+ chelator, i.e., ZnAF-2DA into the SNpc. Furthermore, loss of nigrostriatal dopaminergic neurons induced with a low dose of PQ, which did not induce any behavioral abnormality, was completely blocked by coinjection of ZnAF-2DA. The present study indicates that rapid influx of extracellular Zn2+ into dopaminergic neurons via AMPA receptor activation, which is initially induced by PQ-mediated ROS production in the SNpc, induces nigrostriatal dopaminergic degeneration, resulting in PQ-induced PD in rats. Intracellular Zn2+ dysregulation in dopaminergic neurons is the cause of PQ-induced pathogenesis in the SNpc, and the block of intracellular Zn2+ toxicity leads to defending PQ-induced pathogenesis.








Similar content being viewed by others
References
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123–144
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535
Zhai S, Tanimura A, Graves SM, Shen W, Surmeier DJ (2017) Striatal synapses, circuits, and Parkinson’s disease. Curr Opin Neurobiol 48:9–16
Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS et al (2011) Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 119:866–872
Ritz BR, Paul KC, Bronstein JM (2016) Of pesticides and men: a California story of genes and environment in Parkinson’s disease. Curr Environ Health Rep 3:40–52
Hertzman C, Wiens M, Bowering D, Snow B, Calne D (1990) Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med 17:349–355
Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC (1997) Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology 48:1583–1588
Zhang XF, Thompson M, Xu YH (2016) Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab Investig 96:496–507
Vaccari C, El Dib R, de Camargo JLV (2017) Paraquat and Parkinson’s disease: a systematic review protocol according to the OHAT approach for hazard identification. Syst Rev 6:98
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER (2006) Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49:603–615
Drechsel DA, Patel M (2008) Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med 44:1873–1886
Shimizu K, Matsubara K, Ohtaki K, Fujimaru S, Saito O, Shiono H (2003) Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res 976:243–252
Ambrosi G, Cerri S, Blandini F (2014) A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J Neural Transm (Vienna) 121:849–859
Oster S, Radad K, Scheller D, Hesse M, Balanzew W, Reichmann H, Gille G (2014) Rotigotine protects against glutamate toxicity in primary dopaminergic cell culture. Eur J Pharmacol 724:31–42
Weiss JH, Sensi SL (2000) Ca2+-Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci 23:365–371
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Sensi SL, Paoletti P, Bush AI, Sekler I (2009) Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 10:780–791
Pochwat B, Nowak G, Szewczyk B (2015) Relationship between zinc (Zn (2+)) and glutamate receptors in the processes underlying neurodegeneration. Neural Plast 2015:591563
Takeda A, Tamano H (2016) Insight into cognitive decline from Zn2+ dynamics through extracellular signaling of glutamate and glucocorticoids. Arch Biochem Biophys 611:93–99
Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272:1013–1016
Liu S, Lau L, Wei J, Zhu D, Zou S, Sun HS, Fu Y, Liu F et al (2004) Expression of Ca(2+)-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron 43:43–55
Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV (2005) Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against globalischemia-induced death. Proc Natl Acad Sci U S A 102:12230–12235
Frederickson CJ (1989) Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol 31:145–238
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y, Balaji RV, Masalha R et al (2006) Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol 198:285–293
Tamano H, Nishio R, Shakushi Y, Sasaki M, Koike Y, Osawa M, Takeda A (2017) In vitro and in vivo physiology of low nanomolar concentrations of Zn2+ in artificial cerebrospinal fluid. Sci Rep 7:42897
Hirano T, Kikuchi K, Urano Y, Nagano T (2002) Improvement and biological applications of fluorescent probes for zinc, ZnAFs. J Am Chem Soc 124:6555–6562
Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N et al (2002) Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. J Cell Biol 158:215–220
Kumar A, Singh BK, Ahmad I, Shukla S, Patel DK, Srivastava G, Kumar V, Pandey HP et al (2012) Involvement of NADPH oxidase and glutathione in zinc-induced dopaminergic neurodegeneration in rats: similarity with paraquat neurotoxicity. Brain Res 1438:48–64
Kumar A, Ahmad I, Shukla S, Singh BK, Patel DK, Pandey HP, Singh C (2010) Effect of zinc and paraquat co-exposure on neurodegeneration: modulation of oxidative stress and expression of metallothioneins, toxicant responsive and transporter genes in rats. Free Radic Res 44:950–965
Beal MF (1998) Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann Neurol 44:S110–S114
Zhang L, Dawson VL, Dawson TM (2006) Role of nitric oxide in Parkinson’s disease. Pharmacol Ther 109:33–41
Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW (1997) Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci 15:9554–9564
Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR (2008) Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Physiol Cell Physiol 294:C726–C742
Suzuki M, Fujise Y, Tsuchiya Y, Tamano H, Takeda A (2015) Excess influx of Zn2+ into dentate granule cells affects object recognition memory via attenuated LTP. Neurochem Int 87:60–65
Takeda A, Tamano H, Hisatsune M, Murakami T, Nakada H, Fujii H (2018) Maintained LTP and memory are lost by Zn2+ influx into dentate granule cells, but not Ca2+ influx. Mol Neurobiol 55:1498–1508
Takeda A, Koike Y, Osawa M, Tamano H (2018) Characteristic of extracellular Zn2+ influx in the middle-aged dentate gyrus and its involvement in attenuation of LTP. Mol Neurobiol 55:2185–2195
Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV (2003) Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci U S A 100:2906–2910
Weiss JH (2011) Ca permeable AMPA channels in diseases of the nervous system. Front Mol Neurosci 4:42
Tamano H, Nishio R, Morioka H, Takeda A (2018) Extracellular Zn2+ influx into nigral dopaminergic neurons plays a key role for pathogenesis of 6-hydroxydopamine-induced Parkinson’s disease in rats. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1075-z
Sheline CT, Cai AL, Zhu J, Shi C (2010) Serum or target deprivation-induced neuronal death causes oxidative neuronal accumulation of Zn2+ and loss of NAD+. Eur J Neurosci 32:894–904
Medvedeva YV, Ji SG, Yin HZ, Weiss JH (2017) Differential vulnerability of CA1 versus CA3 pyramidal neurons after ischemia: possible relationship to sources of Zn2+ accumulation and its entry into and prolonged effects on mitochondria. J Neurosci 37:726–737
Chen Y, Irie Y, Keung WM, Maret W (2002) S-Nitrosothiols react preferentially with zinc thiolate clusters of metallothionein III through transnitrosation. Biochemistry 41:8360–8367
Abiria SA, Krapivinsky G, Sah R, Santa-Cruz AG, Chaudhuri D, Zhang J, Adstamongkonkul P, DeCaen PG et al (2017) TRPM7 senses oxidative stress to release Zn2+ from unique intracellular vesicles. Proc Natl Acad Sci U S A 114:E6079–E6088
Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK (2007) Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J Neurosci 27:6914–6922
Peng J, Stevenson FF, Oo ML, Andersen JK (2009) Iron-enhanced paraquat-mediated dopaminergic cell death due to increased oxidative stress as a consequence of microglial activation. Free Radic Biol Med 46:312–320
Takeda A, Nakamura M, Fujii H, Uematsu C, Minamino T, Adlard PA, Bush AI, Tamano T (2014) Amyloid β-mediated Zn2+ influx into dentate granule cells transiently induces a short-term cognitive deficit. PLoS One 9:e115923
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the University of Shizuoka that refer to the American Association for Laboratory Animals Science and the guidelines laid down by the NIH (NIH Guide for the Care and Use of Laboratory Animals) in the USA. The ethics committee of the University of Shizuoka has approved all experimental protocols (approval number, 136043).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tamano, H., Morioka, H., Nishio, R. et al. Blockade of Rapid Influx of Extracellular Zn2+ into Nigral Dopaminergic Neurons Overcomes Paraquat-Induced Parkinson’s Disease in Rats. Mol Neurobiol 56, 4539–4548 (2019). https://doi.org/10.1007/s12035-018-1398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1398-9