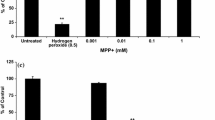
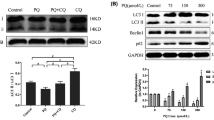
Abstract
On the basis of the evidence that paraquat (PQ)-induced extracellular Zn2+ influx causes PQ-induced pathogenesis in the substantia nigra pars compacta (SNpc) of rats, we postulated that the transient receptor potential melastatin 2 (TRPM2) cation channels activated with PQ-induced reactive oxygen species (ROS) are linked with extracellular glutamate accumulation in the SNpc, followed by age-related intracellular Zn2+ dysregulation. Presynaptic activity (glutamate exocytosis), which was determined with FM4-64, was enhanced in the SNpc after exposure to PQ, and the enhancement was inhibited in the presence of N-(p-amylcinnamoyl)anthranilic acid (ACA), a blocker of TRPM2 cation channels, suggesting that PQ-induced ROS enhances presynaptic activity in the SNpc, probably via TRPM2 channel activation. Extracellular glutamate concentration in the SNpc was increased almost to the same extent under the SNpc perfusion with PQ of young and aged rats, and was suppressed by co-perfusion with ACA, suggesting that PQ-induced TRPM2 cation channel activation enhances glutamate exocytosis in the SNpc. Interestingly, PQ more markedly increased intracellular Zn2+ in the aged SNpc, which was also blocked by co-injection of ACA and CaEDTA, an extracellular Zn2+ chelator. Loss of nigrostriatal dopaminergic neurons was more severely increased in aged rats and completely blocked by co-injection of PQ and CaEDTA into the SNpc. The present study indicates that rapid influx of extracellular Zn2+ into dopaminergic neurons via PQ-induced TRPM2 cation channel activation accelerates nigrostriatal dopaminergic degeneration in aged rats. It is likely that vulnerability to PQ-induced pathogenesis in the aged SNpc is due to accelerated intracellular Zn2+ dysregulation.






Similar content being viewed by others
References
Tanner CM (1989) The role of environmental toxins in the etiology of Parkinson’s disease. Trends Neurosci 12:49–54
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123–144
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535
Ritz BR, Paul KC, Bronstein JM (2016) Of pesticides and men: a California story of genes and environment in Parkinson’s disease. Curr Environ Health Rep 3:40–52
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Lang AE, Lozano AM (1998) Parkinson’s disease. First of two parts. N Engl J Med 339:1044–1053
Irwin I, Finnegan KT, Delanney LE, Di Monte D, Langston JW (1992) The relationships between aging, monoamine oxidase, striatal dopamine and the effects of MPTP in C57BL/6 mice: a critical reassessment. Brain Res 572:224–231
Irwin I, DeLanney LE, Langston JW (1993) MPTP and aging. Studies in the C57BL/6 mouse. Adv Neurol 60:197–206
Hertzman C, Wiens M, Bowering D, Snow B, Calne D (1990) Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med 17:349–355
Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC (1997) Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology 48:1583–1588
Zhang XF, Thompson M, Xu YH (2016) Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab Investig 96:496–507
Vaccari C, El Dib R, de Camargo JLV (2017) Paraquat and Parkinson's disease: a systematic review protocol according to the OHAT approach for hazard identification. Syst Rev 6:98
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER (2006) Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49:603–615
Drechsel DA, Patel M (2008) Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic Biol Med 44:1873–1886
Shimizu K, Matsubara K, Ohtaki K, Fujimaru S, Saito O, Shiono H (2003) Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res 976:243–252
Tamano H, Morioka H, Nishio R, Takeuchi A, Takeda A (2018) Blockade of rapid influx of extracellular Zn2+ into nigral dopaminergic neurons overcomes Paraquat-induced Parkinson’s disease in rats. Mol Neurobiol 56:4539–4548. https://doi.org/10.1007/s12035-018-1398-9
Tamano H, Morioka H, Nishio R, Takeuchi A, Takeda A (2018) AMPA-induced extracellular Zn2+ influx into nigral dopaminergic neurons causes movement disorder in rats. NeuroToxicology 69:23–28
Tamano H, Nishio R, Morioka H, Takeda A (2018) Extracellular Zn2+ influx into nigral dopaminergic neurons plays a key role for pathogenesis of 6-hydroxydopamine-induced Parkinson’s disease in rats. Mol Neurobiol 56:435–443. https://doi.org/10.1007/s12035-018-1075-z
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y, Balaji RV, Masalha R et al (2006) Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol 198:285–293
Tamano H, Nishio R, Shakushi Y, Sasaki M, Koike Y, Osawa M, Takeda A (2017) In vitro and in vivo physiology of low nanomolar concentrations of Zn2+ in artificial cerebrospinal fluid. Sci Rep 7:42897
Takeda A, Koike Y, Osawa M, Tamano H (2018) Characteristic of extracellular Zn2+ influx in the middle-aged dentate gyrus and its involvement in attenuation of LTP. Mol Neurobiol 55:2185–2195
Kraft R, Grimm C, Frenzel H, Harteneck C (2006) Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol 148:264–273
Harteneck C, Frenzel H, Kraft R (2007) N-(p-amylcinnamoyl)anthranilic acid (ACA): a phospholipase A(2) inhibitor and TRP channel blocker. Cardiovasc Drug Rev 25:61–75
Turlova E, Feng ZP, Sun HS (2018) The role of TRPM2 channels in neurons, glial cells and the blood-brain barrier in cerebral ischemia and hypoxia. Acta Pharmacol Sin 39:713–721
Hirano T, Kikuchi K, Urano Y, Nagano T (2002) Improvement and biological applications of fluorescent probes for zinc, ZnAFs. J Am Chem Soc 124:6555–6562
Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N et al (2002) Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. J Cell Biol 158:215–220
Klingauf J, Kavalali ET, Tsien RW (1998) Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature 394:581–585
Zakharenko SS, Zablow L, Siegelbaum SA (2001) Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat Neurosci 4:711–717
Cheramy A, Leviel V, Glowinski J (1981) Dendritic release of dopamine in the substantia nigra. Nature 289:537–542
Chen BT, Rice ME (2002) Synaptic regulation of somatodendritic dopamine release by glutamate and GABA differs between substantia nigra and ventral tegmental area. J Neurochem 81:158–169
Rice ME, Patel JC, Cragg SJ (2011) Dopamine release in the basal ganglia. Neuroscience 198:112–137
Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB (1991) Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem 56:978–982
Riederer P, Dirr A, Goetz M, Sofic E, Jellinger K, Youdim MB (1992) Distribution of iron in different brain regions and subcellular compartments in Parkinson’s disease. Ann Neurol 32:S101–S104
Jellinger KA, Kienzl E, Rumpelmaier G, Paulus W, Riederer P, Stachelberger H, Youdim MB, Ben-Shachar D (1993) Iron and ferritin in substantia nigra in Parkinson’s disease. Adv Neurol 60:267–272
Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK (2007) Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J Neurosci 27:6914–6922
Kumar A, Singh BK, Ahmad I, Shukla S, Patel DK, Srivastava G, Kumar V, Pandey HP et al (2012) Involvement of NADPH oxidase and glutathione in zinc-induced dopaminergic neurodegeneration in rats: similarity with paraquat neurotoxicity. Brain Res 1438:48–64
Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW (1997) Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci 15:9554–9564
Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR (2008) Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Phys Cell Phys 294:C726–C742
Clapham DE, Julius D, Montell C, Schultz G (2005) International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57:427–450
Kita H, Kitai ST (1987) Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol 260:435–452
Ambrosi G, Cerri S, Blandini F (2014) A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J Neural Transm (Vienna) 121:849–859
Chatha BT, Bernard V, Streit P, Bolam JP (2000) Synaptic localization of ionotropic glutamate receptors in the rat substantia nigra. Neuroscience 101:1037–1051
Schmidt WJ, Bubser M, Hauber W (1990) Excitatory amino acids and Parkinson’s disease. Trends Neurosci 13:46–47
Difazio MC, Hollingsworth Z, Young AB, Penney JB Jr (1992) Glutamate receptors in the substantia nigra of Parkinson’s disease brains. Neurology 42:402–406
Blandini F, Porter RH, Greenamyre JT (1996) Glutamate and Parkinson’s disease. Mol Neurobiol 12:73–94
Rodriguez MC, Obeso JA, Olanow CW (1998) Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann Neurol 44:S175–S188
Izumi Y, Yamamoto N, Matsuo T, Wakita S, Takeuchi H, Kume T, Katsuki H, Sawada H et al (2009) Vulnerability to glutamate toxicity of dopaminergic neurons is dependent on endogenous dopamine and MAPK activation. J Neurochem 110:745–755
Sun Y, Sukumaran P, Selvaraj S, Cilz NI, Schaar A, Lei S, Singh BB (2018) TRPM2 promotes neurotoxin MPP+/MPTP-induced cell death. Mol Neurobiol 55:409–420
Jia Y, Jeng JM, Sensi SL, Weiss JH (2002) Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J Physiol 543:35–48
Kwak S, Weiss JH (2006) Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol 16:281–287
Takeda A, Tamano H, Murakami T, Nakada H, Minamino T, Koike Y (2018) Weakened intracellular Zn2+-buffering in the aged dentate gyrus and its involvement in erasure of maintained LTP. Mol Neurobiol 55:3856–3865
Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA (2003) Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J Neurosci 18:589–600
Date I, Felten DL, Felten SY (1990) Long-term effect of MPTP in the mouse brain in relation to aging: neurochemical and immunocytochemical analysis. Brain Res 519:266–276
Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135–172
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamano, H., Nishio, R., Morioka, H. et al. Paraquat as an Environmental Risk Factor in Parkinson’s Disease Accelerates Age-Related Degeneration Via Rapid Influx of Extracellular Zn2+ into Nigral Dopaminergic Neurons. Mol Neurobiol 56, 7789–7799 (2019). https://doi.org/10.1007/s12035-019-01642-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01642-5