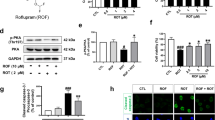
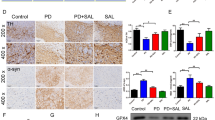
Abstract
Nuclear receptor related 1 (Nurr1) orphan receptor has emerged as a promising contender in ameliorating Parkinson’s disease; thus, finding a suitable activator of Nurr1 receptor is an attracting target for treating PD. Cilostazol, a phosphodiesterase-3 inhibitor, recently showed a favorable neuroprotective activity in multiple devastating central disorders, yet the possible antiparkinsonian activity of the drug has not been fully elucidated. Thus, the aim of this study is to explore the neuroprotective effect of cilostazol in rotenone-induced PD model in rats. Cilostazol successfully upregulated Nurr1 expression in PD rats, which resulted in successful preservation of the dopaminergic neuron functionality and integrity as verified by the marked improvement of motor performance in rotarod and open field tests, as well as the increased striatal tyrosine hydroxylase content. Moreover, cilostazol revealed an anti-inflammatory activity as manifested by hampering the global controller of inflammatory signaling pathway, nuclear factor-kappa B, together with its downstream pro-inflammatory cytokines, namely tumor necrosis factor-alpha and interleukin-1 beta, via Nurr-1 upregulation and glycogen synthase kinase 3 beta GSK-3β inhibition. In turn, the increase in GSK-3β inhibited form suppressed the measured downstream apoptotic biomarkers, viz. cytochrome C and caspase-3. Remarkably, cilostazol enhanced autophagy as depicted by hampering both LC3-II and P62 levels possibly through the prominent rise in sirtuin 1 level. In conclusion, cilostazol could be a promising candidate for PD treatment through modulating Nurr1 expression, as well as SIRT-1/autophagy, and GSK-3β/apoptosis cross-regulation.

In the rat rotenone model of Parkinson’s disease (PD), Nurr1 expression was downregulated, GSK-3β was activated, and autophagic flux was inhibited. Those deleterious effects were associated with deteriorated motor functions, striatal TH content, enhanced inflammatory state, and apoptotic cascade. Cilostazol, a phosphodiesterase-3 inhibitor, exerted a potential protective effect against PD through Nurr1 enhancement, GSK-3β/apoptosis modulation, and SIRT-1/autophagy enhancement. Nurr1 nuclear receptor related 1, TH tyrosine hydroxylase, NF-κB nuclear factor κB, TNFα tumor necrosis factor alpha, ILs interleukins, GSK-3β glycogen synthase kinase 3 beta, SIRT-1 sirtuin 1.




Similar content being viewed by others
References
Alexander GE (2004) Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci 6(3):259–280
Mullin S (2015) Pathogenic mechanisms of neurodegeneration in Parkinson disease. Neurol Clin 33(1):1–17. https://doi.org/10.1016/j.ncl.2014.09.010
Yacoubian TA, Standaert DG (2009) Targets for neuroprotection in Parkinson’s disease. Biochim Biophys Acta 1792(7):676–687. https://doi.org/10.1016/j.bbadis.2008.09.009
Jenner P (2013) Wearing off, dyskinesia, and the use of continuous drug delivery in Parkinson’s disease. Neurol Clin 31(3):17–35. https://doi.org/10.1016/j.ncl.2013.04.010
Kim C, Lee PKJ, Leblanc P (2016) Correlation between orphan nuclear receptor Nurr1 expression and amyloid deposition in 5XFAD mice, an animal model of Alzheimer’s disease. J Neurochem 132(2):254–262. https://doi.org/10.1111/jnc.12935
Smith GA, Rocha EM, Rooney T, Barneoud P, McLean JR, Beagan J, Osborn T, Coimbra M et al (2015) A Nurr1 agonist causes neuroprotection in a Parkinson’s disease lesion model primed with the toll-like receptor 3 dsRNA inflammatory stimulant poly(I:C). PLoS One 10(3):e0121072. https://doi.org/10.1371/journal.pone.0121072
Saijo K, Winner B, Carson CT et al (2009) A Nurr1/CoREST transexpression pathway attenuates neurotoxic inflammation in activated microglia and astrocytes. Cell 137:47–59. https://doi.org/10.1016/j.cell.2009.01.038.A
Yuan YH, Sun JD, Wu MM et al (2013) Rotenone could activate microglia through NFkB associated pathway. Neurochem Res 38(8):1553–1560. https://doi.org/10.1007/s11064-013-1055-7
Blaudin de Thé FX, Rekaik H, Prochiantz A, et al (2016) Neuroprotective transcription factors in animal models of Parkinson disease. Neural Plast 2016:6097107. https://doi.org/10.1155/2016/6097107
Chiara F, Rasola A (2013) GSK-3 and mitochondria in cancer cells. Front Oncol 3:16. https://doi.org/10.3389/fonc.2013.00016
Li DAWEI, Liu ZHIQ, Chen WEI et al (2014) Association of glycogen synthase kinase-3β with Parkinson’s disease (review). Mol Med Rep 9(6):2043–2050. https://doi.org/10.3892/mmr.2014.2080
King TAJD, Clodfelder-miller B, Barksdale KA, Bijur GN (2008) Unregulated mitochondrial GSK3β activity results in NADH:ubiquinone oxidoreductase deficiency. Neurotox Res 14(4):367–382. https://doi.org/10.1007/BF03033861
Ghavami S, Shojaei S, Yeganeh B et al (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49. https://doi.org/10.1016/j.pneurobio.2013.10.004
Janda E, Lascala A, Carresi C et al (2015) Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2 : implications for neuroprotection. Autophagy 11:1063–1080
Hou Y-S, Guan J-J, Xu H-D, Wu F, Sheng R, Qin ZH (2015) Sestrin2 protects dopaminergic cells against rotenone toxicity through AMPK-dependent autophagy activation. Mol Cell Biol 35(16):2740–2751. https://doi.org/10.1128/MCB.00285-15
Ou X, Lee MR (2014) SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 32(5):1183–1194. https://doi.org/10.1002/stem.1641
Zhang A, Wang H, Qin X et al (2012) Genetic analysis of SIRT1 gene promoter in sporadic Parkinson’s disease. Biochem Biophys Res Commun 422(4):693–696. https://doi.org/10.1016/j.bbrc.2012.05.059
Yang H, Zhang W, Pan H et al (2012) SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One 7(9):e46364. https://doi.org/10.1371/journal.pone.0046364
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5(3):344–352. https://doi.org/10.1002/emmm.201302451
Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T (2011) Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals 19(3):163–174. https://doi.org/10.1159/000328516
Choi JM, Shin HK, Kim KY, Lee JH, Hong KW (2002) Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther 300(3):787–793. https://doi.org/10.1124/jpet.300.3.787
Hase Y, Okamoto Y, Fujita Y et al (2012) Cilostazol, a phosphodiesterase inhibitor, prevents no-re fl ow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol 233(1):523–533. https://doi.org/10.1016/j.expneurol.2011.11.038
Hiramatsu M, Takiguchi O, Nishiyama A, Mori H (2010) Cilostazol prevents amyloid β peptide(25-35)-induced memory impairment and oxidative stress in mice. Br J Pharmacol 161(8):1899–1912. https://doi.org/10.1111/j.1476-5381.2010.01014.x
Park SY, Lee HR, Lee WS et al (2016) Cilostazol modulates autophagic degradation of β-amyloid peptide via SIRT1-coupled LKB1/AMPK α signaling in neuronal. PLoS One 11:e0160620. https://doi.org/10.1371/journal.pone.0160620
Abdelkader NF, Safar MM, Salem HA (2016) Ursodeoxycholic acid ameliorates apoptotic cascade in the rotenone model of Parkinson’s disease: modulation of mitochondrial perturbations. Mol Neurobiol 53(2):810–817. https://doi.org/10.1007/s12035-014-9043-8
Kandil EA, Abdelkader NF, El-Sayeh BM, Saleh S (2016) Imipramine and amitriptyline ameliorate the rotenone model of Parkinson’s disease in rats. Neuroscience 332:26–37. https://doi.org/10.1016/j.neuroscience.2016.06.040
Honda F, Imai H, Ishikawa M, Kubota C (2006) Cilostazol attenuates gray and white matter damage in a rodent model of focal cerebral ischemia. Stroke 37(1):223–229. https://doi.org/10.1161/01.STR.0000196977.76702.6d
Cummins RA, Walsh RN (1976) The open-field. Psychol Bull 83(3):482–504. https://doi.org/10.1037/0033-2909.83.3.482
Jones BJ, Roberts DJ (1968) The quantitative measurement of motor inco-ordination in naive mice using an accelerating rotarod. J Pharm Pharmacol 20(4):302–304. https://doi.org/10.1111/j.2042-7158.1968.tb09743.x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 408(4):402–408. https://doi.org/10.1006/meth.2001.1262
Decressac M, Volakakis N, Björklund A, Perlmann T (2013) NURR1 in Parkinson disease—from pathogenesis to therapeutic potential. Nat Rev Neurol 9(11):629–636. https://doi.org/10.1038/nrneurol.2013.209
Kadkhodaei B, Ito T, Joodmardi E et al (2009) Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci 29(50):15923–15932. https://doi.org/10.1523/JNEUROSCI.3910-09.2009
Zetterstrm RH, Solomin L, Jansson L et al (2016) Dopamine neuron agenesis in Nurr1-deficient mice. Am Assoc Adv Sci 276:248–250
Kim C, Han B, Moon J et al (2015) Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc Natl Acad Sci U S A 112(28):6–11. https://doi.org/10.1073/pnas.1509742112
Zhang L, Cen L, Qu S, Wei L, Mo M, Feng J, Sun C, Xiao Y et al (2016) Enhancing beta-catenin activity via GSK3beta inhibition protects PC12 cells against rotenone toxicity through Nurr1 induction. PLoS One 11(4):e0152931. https://doi.org/10.1371/journal.pone.0152931
Zennaro M, Amar L, Skah S et al (2014) WNT/β-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet 23(4):889–905. https://doi.org/10.1093/hmg/ddt484
Episcopo FL, Tirolo C, Caniglia S et al (2014) Targeting Wnt signaling at the neuroimmune interface for dopaminergic neuroprotection/repair in Parkinson’s disease. J Mol Cell Biol 6:13–26. https://doi.org/10.1093/jmcb/mjt053.Targeting
Petit-Paitel A, Brau F, Cazareth J, Chabry J (2009) Involvment of cytosolic and mitochondrial GSK-3β in mitochondrial dysfunction and neuronal cell death of MPTP/MPP+-treated neurons. PLoS One 4(5):e5491. https://doi.org/10.1371/journal.pone.0005491
Chen YY, Chen G, Fan Z et al (2008) GSK3β and endoplasmic reticulum stress mediate rotenone-induced death of SK-N-MC neuroblastoma cells. Biochem Pharmacol 76(1):128–138. https://doi.org/10.1016/j.bcp.2008.04.010
Berthonneche C, Sulpice T, Tanguy S et al (2005) AT1 receptor blockade prevents cardiac dysfunction after myocardial infarction in rats. Cardiovasc Drugs Ther 19(4):251–259. https://doi.org/10.1007/s10557-005-3695-6
Abdel-Raheem IT, Omran GA, Katary MA (2015) Irbesartan, an angiotensin II receptor antagonist, with selective PPAR-gamma-modulating activity improves function and structure of chemotherapy-damaged ovaries in rats. Fundam Clin Pharmacol 29(3):286–298. https://doi.org/10.1111/fcp.12119
Beurel E (2014) Regulation of inflammation and T cells by glycogen synthase kinase-3: Links to mood disorders. Neuroimmunomodulation 21(2-3):140–144. https://doi.org/10.1159/000356550
Ngok-Ngam P, Watcharasit P, Thiantanawat A, Satayavivad J (2013) Pharmacological inhibition of GSK3 attenuates DNA damage-induced apoptosis via reduction of p53 mitochondrial translocation and Bax oligomerization in neuroblastoma SH-SY5Y cells. Cell Mol Biol Lett 18(1):58–74. https://doi.org/10.2478/s11658-012-0039-y
Linseman DA (2004) Glycogen synthase kinase-3 phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 24(44):9993–10002. https://doi.org/10.1523/JNEUROSCI.2057-04.2004
King TD, Bijur GN, Jope RS (2001) Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3 b and attenuated by lithium. Brain Res 919(1):106–114. https://doi.org/10.1016/S0006-8993(01)03005-0
Wang Z, Havasi A, Gall J et al (2010) GSK3β promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 21(2):284–294. https://doi.org/10.1681/ASN.2009080828
Lee JH, Park SY, Shin HK, Kim CD, Lee WS, Hong KW (2008) Protective effects of cilostazol against transient focal cerebral ischemia and chronic cerebral hypoperfusion injury. CNS Neurosci Ther 14(2):143–152. https://doi.org/10.1111/j.1527-3458.2008.00042.x
Tian CJ, Kim YJ, Kim SW, Lim HJ, Kim YS, Choung YH (2013) A combination of cilostazol and Ginkgo biloba extract protects against cisplatin-induced Cochleo-vestibular dysfunction by inhibiting the mitochondrial apoptotic and ERK pathways. Cell Death Dis 4(2):e509. https://doi.org/10.1038/cddis.2013.33
Hayashi H, Sudo T (2009) Effects of the cAMP-elevating agents cilostamide, cilostazol and forskolin on the phosphorylation of Akt and GSK-3 β in platelets. J Thromb Haemost 102:327–335. https://doi.org/10.1160/TH08-12-0781
Li M, Wang X, Meintzer MKAY et al (2000) Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3β. Mol Cell Biol 20:9356–9363
Xilouri M, Brekk OR, Stefanis L (2016) Autophagy and alpha-synuclein: relevance to Parkinson’s disease and related synucleopathies. Mov Disord 31(2):178–192. https://doi.org/10.1002/mds.26477
Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W (2009) Rapamycin protects against rotenone-induced apoptosis through autophagy Induction. Neuroscience 164(2):541–551. https://doi.org/10.1016/j.neuroscience.2009.08.014
Barth S, Glick D, Macleod KF (2010) Autophagy: assays and artifacts. J Pathol 221(2):117–124. https://doi.org/10.1002/path.2694
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3(6):4–7. https://doi.org/10.4161/auto.4600
Mader BJ, Pivtoraiko VN, Flippo HM, Klocke BJ, Roth KA, Mangieri LR, Shacka JJ (2012) Rotenone inhibits autophagic flux prior to inducing cell death. ACS Chem Neurosci 3(12):1063–1072. https://doi.org/10.1021/cn300145z
Lee IH, Cao L, Mostoslavsky R et al (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105:3374–3379
Hariharan N, Maejima Y, Nakae J et al (2010) Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res 107(12):1470–1482. https://doi.org/10.1161/CIRCRESAHA.110.227371
Lee HR, Shin HK, Park SY, Kim HY, Bae SS, Lee WS, Rhim BY, Hong KW et al (2015) Cilostazol upregulates autophagy via SIRT1 activation : reducing amyloid-β peptide and APP-CTF β levels in neuronal cells. PLoS One 10(8):1–14. https://doi.org/10.1371/journal.pone.0134486
Park S, Ahmad F, Philp A et al (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148:421–433. https://doi.org/10.1016/j.cell.2012.01.017.Park
Gerhart-hines Z, Jr JED, Blättler SM et al (2012) The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+. Mol Cell 44:851–863. https://doi.org/10.1016/j.molcel.2011.12.005.The
Wang Z, Zhang L, Liang Y, Zhang C, Xu Z, Zhang L, Fuji R, Mu W et al (2015) Cyclic AMP mimics the anti-ageing effects of calorie restriction by. Nat Publ Gr 5(1):1–10. https://doi.org/10.1038/srep12012
Parr C, Carzaniga R, Gentleman SM et al (2012) Glycogen synthase kinase 3 inhibition promotes lysosomal biogenesis and autophagic degradation of the amyloid-β precursor protein. Mol Cell Biol 32(21):4410–4418. https://doi.org/10.1128/MCB.00930-12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hedya, S.A., Safar, M.M. & Bahgat, A.K. Cilostazol Mediated Nurr1 and Autophagy Enhancement: Neuroprotective Activity in Rat Rotenone PD Model. Mol Neurobiol 55, 7579–7587 (2018). https://doi.org/10.1007/s12035-018-0923-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0923-1