Abstract
Neurogenesis generates fledgling neurons that mature to form an intricate neuronal circuitry. The delusion on adult neurogenesis was far resolved in the past decade and became one of the largely explored domains to identify multifaceted mechanisms bridging neurodevelopment and neuropathology. Neurogenesis encompasses multiple processes including neural stem cell proliferation, neuronal differentiation, and cell fate determination. Each neurogenic process is specifically governed by manifold signaling pathways, several growth factors, coding, and non-coding RNAs. A class of small non-coding RNAs, microRNAs (miRNAs), is ubiquitously expressed in the brain and has emerged to be potent regulators of neurogenesis. It functions by fine-tuning the expression of specific neurogenic gene targets at the post-transcriptional level and modulates the development of mature neurons from neural progenitor cells. Besides the commonly discussed intrinsic factors, the neuronal morphogenesis is also under the control of several extrinsic temporal cues, which in turn are regulated by miRNAs. This review enlightens on dicer controlled switch from neurogenesis to gliogenesis, miRNA regulation of neuronal maturation and the differential expression of miRNAs in response to various extrinsic cues affecting neurogenesis.

Similar content being viewed by others
References
Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A 96:11619–11624
Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6:777–788
Kintner C (2002) Neurogenesis in embryos and in adult neural stem cells. J Neurosci 22:639–643
Urbán N, Guillemot F (2014) Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci 8:396. doi:10.3389/fncel.2014.00396
Ji F, Lv X, Jiao J (2013) The role of microRNAs in neural stem cells and neurogenesis. J Genet Genomics 40:61–66. doi:10.1016/j.jgg.2012.12.008
Asuelime GE, Shi Y (2012) The little molecules that could: a story about microRNAs in neural stem cells and neurogenesis. Front Neurosci 6:176. doi:10.3389/fnins.2012.00176
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Manyroads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11:228–234. doi:10.1038/ncb0309-228
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15:509–524. doi:10.1038/nrm3838
Shi Y, Zhao X, Hsieh J, Wichterle H, Impey S, Banerjee S, Neveu P, Kosik KS (2010) MicroRNA regulation of neural stem cells and neurogenesis. J Neurosci 30:14931–14936. doi:10.1523/JNEUROSCI.4280-10.2010
La Torre A, Georgi S, Reh TA (2013) Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci U S A 110:E2362–E2370. doi:10.1073/pnas
Ambros V (2011) MicroRNAs and developmental timing. Curr Opin Genet Dev 21:511–517. doi:10.1016/j.gde.2011.04.003
Krichevsky AM, Sonntag KC, Isacson O, Kosik KS (2006) Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 24:857–864
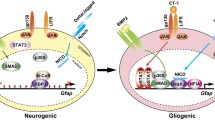
Zheng K, Li H, Zhu Y, Zhu Q, Qiu M (2010) MicroRNAs are essential for the developmental switch from neurogenesis to gliogenesis in the developing spinal cord. J Neurosci 30:8245–8250. doi:10.1523/JNEUROSCI.1169-10.2010
McLoughlin HS, Fineberg SK, Ghosh LL, Tecedor L, Davidson BL (2012) Dicer is required for proliferation, viability, migration and differentiation in corticoneurogenesis. Neuroscience 223:285–295. doi:10.1016/j.neuroscience.2012.08.009
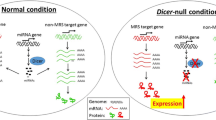
Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K (2005) Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 19:489–501
Martino S, di Girolamo I, Orlacchio A, Datti A, Orlacchio A (2009) MicroRNA implications across neurodevelopment and neuropathology. J Biomed Biotechnol 654346 doi:10.1155/2009/654346
Andersson T, Rahman S, Sansom SN, Alsiö JM, Kaneda M, Smith J, O’Carroll D, Tarakhovsky A et al (2010) Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs. PLoS One 5, e13453. doi:10.1371/journal.pone.0013453
Kawase-Koga Y, Otaegi G, Sun T (2009) Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev Dyn 238:2800–2812. doi:10.1002/dvdy.22109
Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM (2008) Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci Off J Soc Neurosci 28:4322–4330. doi:10.1523/JNEUROSCI.4815-07.2008
Makeyev EV, Zhang J, Carrasco MA, Maniatis T (2007) The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27:435–448. doi:10.1016/j.molcel.2007.07.015
Li Q, Bian S, Hong J, Kawase-Koga Y, Zhu E, Zheng Y, Yang L, Sun T (2011) Timing specific requirement of microRNA function is essential for embryonic and postnatal hippocampal development. PLoS One 6, e26000. doi:10.1371/journal.pone.0026000
De PietriTonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB (2008) miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development 135:3911–3921. doi:10.1242/dev.025080
Nowakowski TJ, Mysiak KS, Pratt T, Price DJ (2011) Functional Dicer is necessary for appropriate specification of radial glia during early development of mouse telencephalon. PLoS One 6, e23013. doi:10.1371/journal.pone.0023013
Saurat N, Andersson T, Vasistha NA, Molnár Z, Livesey FJ (2013) Dicer is required for neural stem cell multipotency and lineage progression during cerebral cortex development. Neural Dev 8:14. doi:10.1186/1749-8104-8-14
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich AA (2007) MicroRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224. doi:10.1126/science.1140481
Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E, McManus MT (2008) Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc Natl Acad Sci U S A 105:5614–5619. doi:10.1073/pnas.0801689105
Schwamborn JC, Berezikov E, Knoblich JA (2009) The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 136:913–925. doi:10.1016/j.cell.2008.12.024
Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N (2012) microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci 15:697–699. doi:10.1038/nn.3082
Strickland IT, Richards L, Holmes FE, Wynick D, Uney JB, Wong LF (2011) Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PLoS One 6, e23423. doi:10.1371/journal.pone.0023423
Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, Zhang ZG (2013) The microRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci 33:6885–6894. doi:10.1523/JNEUROSCI.5180-12.2013
Wu D, Murashov AK (2013) MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front Mol Neurosci 6:35. doi:10.3389/fnmol.2013.00035
Lu CS, Zhai B, Mauss A, Landgraf M, Gygi S, Van Vactor D (2014) MicroRNA-8 promotes robust motor axon targeting by coordinate regulation of cell adhesion molecules during synapse development. Philos Trans R Soc Lond B Biol Sci 369:20130517. doi:10.1098/rstb.2013.0517
Agostini M, Tucci P, Killick R, Candi E, Sayan BS, Rivetti di Val Cervo P, Nicotera P, McKeon F et al (2011) Neuronal differentiation by TAp73 is mediated by microRNA-34a regulation of synaptic protein targets. Proc Natl Acad Sci U S A 108:21093–21098. doi:10.1073/pnas
Zhang J, Zhang J, Liu LH, Zhou Y, Li YP, Shao ZH, Wu YJ, Li MJ et al (2011) Effects of miR-541 on neurite outgrowth during neuronal differentiation. Cell Biochem Funct 29:279–286. doi:10.1002/cbf.1747
Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K et al (2008) An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A 105:9093–9098. doi:10.1073/pnas.0803072105
Siegel G, Saba R, Schratt G (2011) microRNAs in neurons: manifold regulatory roles at the synapse. Curr Opin Genet Dev 21:491–497. doi:10.1016/j.gde.2011.04.008
Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W et al (2010) Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol 189:127–141. doi:10.1083/jcb.200908151
Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Gou W, Pathania M, Teng ZQ, Luo Y et al (2010) MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase Mind Bomb-1. Stem Cells 28:1060–1070. doi:10.1002/stem.431
Kole AJ, Swahari V, Hammond SM, Deshmukh M (2011) miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev 25:125–130. doi:10.1101/gad.1975411
Li H, Mao S, Wang H, Zen K, Zhang C, Li L (2014) MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons. Protein Cell 5:160–169. doi:10.1007/s13238-014-0022-7
Wu D, Raafat A, Pak E, Clemens S, Murashov AK (2012) Dicer-microRNA pathway is critical for peripheral nerve regeneration and functional recovery in vivo and regenerative axonogenesis in vitro. Exp Neurol 233:555–565. doi:10.1016/j.expneurol.2011.11.041
Hancock ML, Preitner N, Quan J, Flanagan JG (2014) MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. J Neurosci 34:66–78. doi:10.1523/JNEUROSCI.3371-13.2014
Natera-Naranjo O, Aschrafi A, Gioio AE, Kaplan BB (2010) Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA 16:1516–1529. doi:10.1261/rna.1833310
Sasaki Y, Gross C, Xing L, Goshima Y, Bassell GJ (2014) Identification of axon-enriched microRNAs localized to growth cones of cortical neurons. Dev Neurobiol 74:397–406. doi:10.1002/dneu.22113
Hong J, Zhang H, Kawase-Koga Y, Sun T (2013) MicroRNA function is required for neurite outgrowth of mature neurons in the mouse postnatal cerebral cortex. Front Cell Neurosci 7:151. doi:10.3389/fncel.2013.00151
Hoshino N, Vatterott P, Egwiekhor A, Rochlin MW (2010) Brain-derived neurotrophic factor attracts geniculate ganglion neurites during embryonic targeting. Dev Neurosci 32:184–196. doi:10.1159/000313902
Panagiotaki N, Dajas-Bailador F, Amaya E, Papalopulu N, Dorey K (2010) Characterisation of a new regulator of BDNF signalling, Sprouty3, involved in axonal morphogenesis in vivo. Development 137:4005–4015. doi:10.1242/dev.053173
Santos AR, Comprido D, Duarte CB (2010) Regulation of local translation at the synapse by BDNF. Prog Neurobiol 92:505–516. doi:10.1016/j.pneurobio.2010.08.004
Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A et al (2008) MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell 19:3272–3282. doi:10.1091/mbc.E08-02-0159
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L et al (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963–966. doi:10.1126/science.1161566
Nampoothiri SS, Menon HV, Das D, G K R (2015) ISCHEMIRs: finding a way through the obstructed cerebral arteries. Curr Drug Targets
Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK et al (2010) PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 13:1075–1081. doi:10.1038/nn.2603
Wheeler G, Ntounia-Fousara S, Granda B, Rathjen T, Dalmay T (2006) Identification of new central nervous system specific mouse microRNAs. FEBS Lett 580:2195–2200. doi:10.1016/j.febslet.2006.03.019
Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S et al (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437:1370–1375. doi:10.1038/nature04108
Solberg N, Machon O, Krauss S (2008) Effect of canonical Wnt inhibition in the neurogenic cortex, hippocampus, and premigratory dentate gyrus progenitor pool. Dev Dyn 237:1799–1811. doi:10.1002/dvdy.21586
Hall AC, Lucas FR, Salinas PC (2000) Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100:525–535. doi:10.1016/S0092-8674(00)80689-3
Budnik V, Salinas PC (2011) Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol 21:151–159. doi:10.1016/j.conb.2010.12.002
Pujol F, Kitabgi P, Boudin H (2005) The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J Cell Sci 118:1071–1080. doi:10.1242/jcs.01694
Cheng H, Zhou L, Li B, Zhu M, Too HP, Choi WK (2014) Nano-topology guided neurite outgrowth in PC12 cells is mediated by miRNAs. Nanomedicine 10:1871–1875. doi:10.1016/j.nano.2014.07.011
Smalheiser NR, Lugli G (2009) microRNA regulation of synaptic plasticity. Neuromolecular Med 11:133–140. doi:10.1007/s12017-009-8065-2
Lugli G, Torvik VI, Larson J, Smalheiser NR (2008) Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J Neurochem 106:650–661. doi:10.1111/j.1471-4159.2008.05413.x
Nesler KR, Sand RI, Symmes BA, Pradhan SJ, Boin NG, Laun AE, Barbee SA, Gillingwater TH (2013) The miRNA pathway controls rapid changes in activity-dependent synaptic structure at the Drosophila melanogaster neuromuscular junction. PLoS One 8:e68385. doi:10.1371/journal.pone.0068385
Agostini M, Tucci P, Steinert JR, Shalom-Feuerstein R, Rouleau M, Aberdam D, Forsythe ID, Young KW et al (2011) microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A 108:21099–21104. doi:10.1073/pnas
Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S (2005) A cAMP-response element-binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A 102:16426–16431. doi:10.1073/pnas.0508448102
Impey S, Davare M, Lesiak A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR et al (2010) An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci 43:146–156. doi:10.1016/j.mcn.2009.10.005
Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH (2010) microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A 107:20382–20387. doi:10.1073/pnas.1015691107
Hwang J-Y, Aromolaran KA, Zukin RS (2013) Epigenetic mechanisms in stroke and epilepsy. Neuropsychopharmacology 38:167–182. doi:10.1038/npp.2012.134
Wanet A, Tacheny A, Arnould T, Renard P (2012) miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res 40:4742–4753. doi:10.1093/nar/gks151
Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH (2007) Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci 10:1513–1514. doi:10.1038/nn2010
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320:1224–1229. doi:10.1126/science.1153252
Strazisar M, Cammaerts S, van der Ven K, Forero DA, Lenaerts AS, Nordin A, Almeida-Souza L, Genovese G et al (2015) MIR137 variants identified in psychiatric patients affect synaptogenesis and neuronal transmission gene sets. Mol Psychiatry 20:472–481. doi:10.1038/mp.2014.53
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776. doi:10.1038/35037710
Cohen D, Segal M, Reiner O (2008) Doublecortin supports the development of dendritic arbors in primary hippocampal neurons. Dev Neurosci 30:187–199. doi:10.1159/000109862
Sempere LF, Sokol NS, Dubrovsky EB, Berger EM, Ambros V (2003) Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-Complex gene activity. Dev Biol 259:9–18. doi:10.1016/S0012-1606(03)00208-2
Jin H, Kim VN, Hyun S (2012) Conserved microRNA miR-8 controls body size in response to steroid signaling in Drosophila. Genes Dev 26:1427–1432. doi:10.1101/gad.192872.112
Varghese J, Cohen SM (2007) microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev 21:2277–2282. doi:10.1101/gad.439807
Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, Kunugi H, Hashido K (2010) Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience 165:1301–1311. doi:10.1016/j.neuroscience.2009.11.057
Huang YH, Lin YH, Chi HC, Liao CH, Liao CJ, Wu SM, Chen CY, Tseng YH et al (2013) Thyroid hormone regulation of miR-21 enhances migration and invasion of hepatoma. Cancer Res 73:2505–2517. doi:10.1158/0008-5472.CAN-12-2218
Kerek R, Geoffroy A, Bison A, Martin N, Akchiche N, Pourié G, Helle D, Guéant JL et al (2013) Early methyl donor deficiency may induce persistent brain defects by reducing Stat3 signaling targeted by miR-124. Cell Death Dis 4, e755. doi:10.1038/cddis.2013.278
Zheng H, Zeng Y, Chu J, Kam AY, Loh HH, Law PY (2010) Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J Neurosci 30:8102–8110. doi:10.1523/JNEUROSCI.6108-09.2010
Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law P-Y (2010) μ-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol 77:102–109. doi:10.1124/mol.109.060848
Xu C, Zhang Y, Zheng H, Loh HH, Law PY (2014) Morphine modulates mouse hippocampal progenitor cell lineages by upregulating miR-181a level. Stem Cells 32:2961–2972. doi:10.1002/stem.1774
Miranda RC (2012) MicroRNAs and fetal brain development: implications for ethanol teratology during the second trimester period of neurogenesis. Front Genet 3:77. doi:10.3389/fgene.2012.00077
Kucherenko MM, Shcherbata HR (2013) Steroids as external temporal codes act via microRNAs and cooperate with cytokines in differential neurogenesis. Fly (Austin) 7:173–183. doi:10.4161/fly.25241
Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, Champagne DL, Schouten T et al (2009) MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology 150:2220–2228. doi:10.1210/en.2008-1335
Guéant JL, Namour F, Guéant-Rodriguez RM, Daval JL (2013) Folate and fetal programming: a play in epigenomics? Trends Endocrinol Metab 24:279–289. doi:10.1016/j.tem.2013.01.010
Amouzou EK, Chabi NW, Adjalla CE, Rodriguez-Guéant RM, Feillet F, Villaume C, Sanni A, Guéant JL (2004) High prevalence of hyperhomocysteinemia related to folate deficiency and the 677 C—4T mutation of the gene encoding methylenetetrahydrofolatereductase in coastal West Africa. Am J Clin Nutr 79:619–624
McLean E, de Benoist B, Allen LH (2008) Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull 29:S38–S51
Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM (1993) Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 86:703–708
Li M, van der Vaart A (2011) MicroRNAs in addiction: adaptation’s middlemen? Mol Psychiatry 16:1159–1168. doi:10.1038/mp.2011.58
Rodríguez RE (2012) Morphine and microRNA activity: is there a relation with addiction? Front Genet 3:223. doi:10.3389/fgene.2012.00223
Wu Q, Law PY, Wei LN, Loh HH (2008) Posttranscriptional regulation of mouse of μ-opioid receptor (MOR1) via its 3′ untranslated region: a row for microRNA23b. Faseb J 22:4085–4095. doi:10.1096/fj.08-108175
Wu Q, Zhang L, Law PY, Wei LN, Loh HH (2009) Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Mol Pharmacol 75:744–750. doi:10.1124/mol.108.053462
Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD (2011) Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res 35:1928–1937. doi:10.1111/j.1530-0277.2011.01544.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nampoothiri, S.S., Rajanikant, G.K. Decoding the ubiquitous role of microRNAs in neurogenesis. Mol Neurobiol 54, 2003–2011 (2017). https://doi.org/10.1007/s12035-016-9797-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9797-2