Abstract
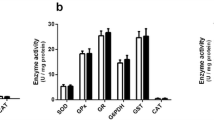
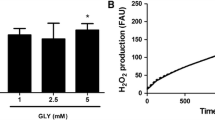
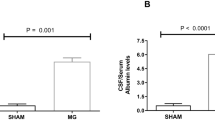
High glycine (GLY) levels have been suggested to induce neurotoxic effects in the central nervous system of patients with nonketotic hyperglycinemia (NKH). Since the mechanisms involved in the neuropathophysiology of NKH are not totally established, we evaluated the effect of a single intracerebroventricular administration of GLY on the content of proteins involved in neuronal damage and inflammatory response, as well as on the phosphorylation of the MAPK p38, ERK1/2, and JNK in rat striatum and cerebral cortex. We also examined glial fibrillary acidic protein (GFAP) staining, a marker of glial reactivity. The parameters were analyzed 30 min or 24 h after GLY administration. GLY decreased Tau phosphorylation in striatum and cerebral cortex 30 min and 24 h after its administration. On the other hand, synaptophysin levels were decreased in striatum at 30 min and in cerebral cortex at 24 h after GLY injection. GLY also decreased the phosphorylation of p38, ERK1/2, and JNK 30 min after its administration in both brain structures. Moreover, GLY-induced decrease of p38 phosphorylation in striatum was attenuated by N-methyl-d-aspartate receptor antagonist MK-801. In contrast, synuclein, NF-κB, iκB, inducible nitric oxide synthase and nitrotyrosine content, and GFAP immunostaining were not altered by GLY infusion. It may be presumed that the decreased phosphorylation of MAPK associated with alterations of markers of neuronal injury induced by GLY may contribute to the neurological dysfunction observed in NKH.










Similar content being viewed by others
References
Heindel W, Kugel H, Roth B (1993) Noninvasive detection of increased glycine content by proton MR spectroscopy in the brains of two infants with nonketotic hyperglycinemia. Am J Neuroradiol 14(3):629–635
Hamosh A, Johnston MV (2001) Non-ketotic hyperglycinemia. In: Scriver CR, Beaudet A, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol editors, 8th edn. McGraw-Hill, New York, pp. 2065–2078
Kure S, Korman SH, Kanno J, Narisawa A, Kubota M, Takayanagi T, Takayanagi M, Saito T et al (2006) Rapid diagnosis of glycine encephalopathy by 13C-glycine breath test. Ann Neurol 59(5):862–867. doi:10.1002/ana.20853
Applegarth DA, Toone JR (2006) Glycine encephalopathy (nonketotic hyperglycinemia): comments and speculations. Am J Med Genet A 140(2):186–188. doi:10.1002/ajmg.a.31030
Van Hove J, Coughlin C II, Scharer G (1993) Glycine encephalopathy. In: Pagon RA, Adam MP, Ardinger HH et al. (eds) Gene Reviews (R). Seattle (WA)
Mourmans J, Majoie CB, Barth PG, Duran M, Akkerman EM, Poll-The BT (2006) Sequential MR imaging changes in nonketotic hyperglycinemia. Am J Neuroradiol 27(1):208–211
Raghavendra S, Ashalatha R, Thomas SV, Kesavadas C (2007) Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology 49(4):299–305. doi:10.1007/s00234-006-0189-6
Shuman RM, Leech RW, Scott CR (1978) The neuropathology of the nonketotic and ketotic hyperglycinemias: three cases. Neurology 28(2):139–146
Hara H, Sukamoto T, Kogure K (1993) Mechanism and pathogenesis of ischemia-induced neuronal damage. Prog Neurobiol 40(6):645–670. doi:10.1016/0301-0082(93)90009-H
Kure S, Tada K, Narisawa K (1997) Nonketotic hyperglycinemia: biochemical, molecular, and neurological aspects. J Hum Genet 42(1):13–22. doi:10.1007/BF02766917
Applegarth DA, Toone JR (2001) Nonketotic hyperglycinemia (glycine encephalopathy): laboratory diagnosis. Mol Genet Metab 74(1–2):139–146. doi:10.1006/mgme.2001.3224
Kono Y, Shigetomi E, Inoue K, Kato F (2007) Facilitation of spontaneous glycine release by anoxia potentiates NMDA receptor current in the hypoglossal motor neurons of the rat. Eur J Neurosci 25(6):1748–1756. doi:10.1111/j.1460-9568.2007.05426.x
Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A (2007) Contribution of endogenous glycine and d-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci 81(9):740–749. doi:10.1016/j.lfs.2007.07.001
Moura AP, Grings M, Dos Santos Parmeggiani B, Marcowich GF, Tonin AM, Viegas CM, Zanatta A, Ribeiro CA et al (2013) Glycine intracerebroventricular administration disrupts mitochondrial energy homeostasis in cerebral cortex and striatum of young rats. Neurotox Res 24(4):502–511. doi:10.1007/s12640-013-9396-1
Leipnitz G, Solano AF, Seminotti B, Amaral AU, Fernandes CG, Beskow AP, Dutra Filho CS, Wajner M (2009) Glycine provokes lipid oxidative damage and reduces the antioxidant defenses in brain cortex of young rats. Cell Mol Neurobiol 29(2):253–261. doi:10.1007/s10571-008-9318-6
Busanello EN, Moura AP, Viegas CM, Zanatta A, da Costa FG, Schuck PF, Wajner M (2010) Neurochemical evidence that glycine induces bioenergetical dysfunction. Neurochem Int 56(8):948–954. doi:10.1016/j.neuint.2010.04.002
Seminotti B, Knebel LA, Fernandes CG, Amaral AU, da Rosa MS, Eichler P, Leipnitz G, Wajner M (2011) Glycine intrastriatal administration induces lipid and protein oxidative damage and alters the enzymatic antioxidant defenses in rat brain. Life Sci 89(7–8):276–281. doi:10.1016/j.lfs.2011.06.013
Moura AP, Parmeggiani B, Grings M, Alvorcem LM, Boldrini RM, Bumbel AP, Motta MM, Seminotti B et al (2015) Intracerebral glycine administration impairs energy and redox homeostasis and induces glial reactivity in cerebral cortex of newborn rats. Mol Neurobiol. doi:10.1007/s12035-015-9493-7
Yang SH, Sharrocks AD, Whitmarsh AJ (2013) MAP kinase signalling cascades and transcriptional regulation. Gene 513(1):1–13. doi:10.1016/j.gene.2012.10.033
Hara MR, Snyder SH (2007) Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol 47:117–141. doi:10.1146/annurev.pharmtox.47.120505.105311
Prentice H, Modi JP, Wu JY (2015) Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxidative Med Cell Longev 2015:964518. doi:10.1155/2015/964518
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Ribeiro CAJ, Grando V, Dutra CS, Wannmacher CMD, Wajner M (2006) Evidence that quinolinic acid severely impairs energy metabolism through activation of NMDA receptors in striatum from developing rats. J Neurochem 99(6):1531–1542. doi:10.1111/j.1471-4159.2006.04199.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Olivera S, Fernandez A, Latini A, Rosillo JC, Casanova G, Wajner M, Cassina P, Barbeito L (2008) Astrocytic proliferation and mitochondrial dysfunction induced by accumulated glutaric acidemia I (GAI) metabolites: possible implications for GAI pathogenesis. Neurobiol Dis 32(3):528–534. doi:10.1016/j.nbd.2008.09.011
Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802(4):396–405. doi:10.1016/j.bbadis.2009.12.009
Poddar R, Paul S (2013) Novel crosstalk between ERK MAPK and p38 MAPK leads to homocysteine-NMDA receptor-mediated neuronal cell death. J Neurochem 124(4):558–570. doi:10.1111/jnc.12102
Terek D, Koroglu OA, Gunes S, Yalaz M, Akisu M, Ucar SK, Gokben S, Coker M et al (2012) Diagnostic tools of metabolic and structural brain disturbances in neonatal non-ketotic hyperglycinemia. Pediatr Int 54(5):717–720. doi:10.1111/j.1442-200X.2012.03591.x
Demirel N, Bas AY, Zenciroglu A, Aydemir C, Kalkanoglu S, Coskun T (2008) Neonatal non-ketotic hyperglycinemia: report of five cases. Pediatr Int 50(1):121–123. doi:10.1111/j.1442-200X.2007.02513.x
Alder J, Kanki H, Valtorta F, Greengard P, Poo MM (1995) Overexpression of synaptophysin enhances neurotransmitter secretion at Xenopus neuromuscular synapses. J Neuros 15(1 Pt 2):511–519
Janz R, Sudhof TC, Hammer RE, Unni V, Siegelbaum SA, Bolshakov VY (1999) Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron 24(3):687–700
Kim E, Sheng M (2004) PDZ domain proteins of synapses. Nat Rev Neurosci 5(10):771–781. doi:10.1038/nrn1517
Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS, Henn FA (2001) Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biol Psychiatry 50(10):809–812
Xu H, He J, Richardson JS, Li XM (2004) The response of synaptophysin and microtubule-associated protein 1 to restraint stress in rat hippocampus and its modulation by venlafaxine. J Neurochem 91(6):1380–1388. doi:10.1111/j.1471-4159.2004.02827.x
Schmitt U, Tanimoto N, Seeliger M, Schaeffel F, Leube RE (2009) Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 162(2):234–243. doi:10.1016/j.neuroscience.2009.04.046
Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ (1997) Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol 56(8):933–944
Terry RD (1998) The cytoskeleton in Alzheimer disease. J Neural Transm Suppl 53:141–145
Kuszczyk M, Gordon-Krajcer W, Lazarewicz JW (2009) Homocysteine-induced acute excitotoxicity in cerebellar granule cells in vitro is accompanied by PP2A-mediated dephosphorylation of tau. Neurochem Int 55(1–3):174–180. doi:10.1016/j.neuint.2009.02.010
Zambrano CA, Egana JT, Nunez MT, Maccioni RB, Gonzalez-Billault C (2004) Oxidative stress promotes tau dephosphorylation in neuronal cells: the roles of cdk5 and PP1. Free Radic Biol Med 36(11):1393–1402. doi:10.1016/j.freeradbiomed.2004.03.007
Kumar P, Jha NK, Jha SK, Ramani K, Ambasta RK (2015) Tau phosphorylation, molecular chaperones, and ubiquitin E3 ligase: clinical relevance in Alzheimer’s disease. J Alzheimers Dis 43(2):341–361. doi:10.3233/JAD-140933
Qi H, Prabakaran S, Cantrelle FX, Chambraud B, Gunawardena J, Lippens G, Landrieu I (2016) Characterization of neuronal tau protein as a target of extracellular signal-regulated kinase. J Biol Chem 291(14):7742–7753. doi:10.1074/jbc.M115.700914
Poddar R, Paul S (2009) Homocysteine-NMDA receptor-mediated activation of extracellular signal-regulated kinase leads to neuronal cell death. J Neurochem 110(3):1095–1106. doi:10.1111/j.1471-4159.2009.06207.x
Subramaniam S, Zirrgiebel U, von Bohlen Und Halbach O, Strelau J, Laliberte C, Kaplan DR, Unsicker K (2004) ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J Cell Biol 165(3):357–369. doi:10.1083/jcb.200403028
Kyosseva SV, Elbein AD, Hutton TL, Griffin ST, Mrak RE, Sturner WQ, Karson CN (2000) Increased levels of transcription factors Elk-1, cyclic adenosine monophosphate response element-binding protein, and activating transcription factor 2 in the cerebellar vermis of schizophrenic patients. Arch Gen Psychiatry 57(7):685–691
Kyosseva SV, Elbein AD, Griffin WS, Mrak RE, Lyon M, Karson CN (1999) Mitogen-activated protein kinases in schizophrenia. Biol Psychiatry 46(5):689–696
Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN (2001) Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem 77(3):916–928
Wang Z, Gu J, Wang X, Xie K, Luan Q, Wan N, Zhang Q, Jiang H et al (2013) Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: the HPA axis, BDNF expression and phosphorylation of ERK. Pharmacol Biochem Behav 112:104–110. doi:10.1016/j.pbb.2013.10.007
Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS (2007) A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry 61(5):661–670. doi:10.1016/j.biopsych.2006.05.047
Kim SH, Markham JA, Weiler IJ, Greenough WT (2008) Aberrant early-phase ERK inactivation impedes neuronal function in fragile X syndrome. Proc Natl Acad Sci U S A 105(11):4429–4434. doi:10.1073/pnas.0800257105
Wang J, Ming H, Chen R, Ju JM, Peng WD, Zhang GX, Liu CF (2015) CIH-induced neurocognitive impairments are associated with hippocampal Ca(2+) overload, apoptosis, and dephosphorylation of ERK1/2 and CREB that are mediated by overactivation of NMDARs. Brain Res 1625:64–72. doi:10.1016/j.brainres.2015.08.012
Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, Legos JJ et al (2001) Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med Res Rev 21(2):129–145
Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF et al (2001) SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther 296(2):312–321
De Montigny A, Elhiri I, Allyson J, Cyr M, Massicotte G (2013) NMDA reduces Tau phosphorylation in rat hippocampal slices by targeting NR2A receptors, GSK3beta, and PKC activities. Neural Plast 2013:261593. doi:10.1155/2013/261593
Fleming LM, Johnson GV (1995) Modulation of the phosphorylation state of tau in situ: the roles of calcium and cyclic AMP. Biochem J 309(Pt 1):41–47
Schroder NW, Opitz B, Lamping N, Michelsen KS, Zahringer U, Gobel UB, Schumann RR (2000) Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J Immunol 165(5):2683–2693
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75(6):639–653. doi:10.1016/j.lfs.2003.10.042
Seminotti B, Ribeiro RT, Amaral AU, da Rosa MS, Pereira CC, Leipnitz G, Koeller DM, Goodman S et al (2014) Acute lysine overload provokes protein oxidative damage and reduction of antioxidant defenses in the brain of infant glutaryl-CoA dehydrogenase deficient mice: a role for oxidative stress in GA I neuropathology. J Neurol Sci 344(1–2):105–113. doi:10.1016/j.jns.2014.06.034
Shoham S, Javitt DC, Heresco-Levy U (1999) High dose glycine nutrition affects glial cell morphology in rat hippocampus and cerebellum. International Journal Neuropsychopharmacology 2(1):35–40. doi:10.1017/S1461145799001285
Ohya Y, Ochi N, Mizutani N, Hayakawa C, Watanabe K (1991) Nonketotic hyperglycinemia: treatment with NMDA antagonist and consideration of neuropathogenesis. Pediatr Neurol 7(1):65–68
Korman SH, Wexler ID, Gutman A, Rolland MO, Kanno J, Kure S (2006) Treatment from birth of nonketotic hyperglycinemia due to a novel GLDC mutation. Ann Neurol 59(2):411–415. doi:10.1002/ana.20759
Schmitt B, Steinmann B, Gitzelmann R, Thun-Hohenstein L, Mascher H, Dumermuth G (1993) Nonketotic hyperglycinemia: clinical and electrophysiologic effects of dextromethorphan, an antagonist of the NMDA receptor. Neurology 43(2):421–424
Acknowledgements
The authors declare that there is no conflict of interest. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX II), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Pró-Reitoria de Pesquisa/Universidade Federal do Rio Grande do Sul (PROPESQ/UFRGS), Financiadora de estudos e projetos (FINEP), Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00, and Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção (INCT-EN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were approved by the local Animal Ethics Commission (Universidade Federal do Rio Grande do Sul) under the number 23787 and the National Animal Rights Regulations (Law 11.794/2008). The guidelines of National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 80–23, revised 1996) and Directive 2010/63/EU were followed. All efforts were made to minimize the number of animals used and their suffering.
Additional information
Alana Pimentel Moura and Belisa Parmeggiani have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Moura, A.P., Parmeggiani, B., Gasparotto, J. et al. Glycine Administration Alters MAPK Signaling Pathways and Causes Neuronal Damage in Rat Brain: Putative Mechanisms Involved in the Neurological Dysfunction in Nonketotic Hyperglycinemia. Mol Neurobiol 55, 741–750 (2018). https://doi.org/10.1007/s12035-016-0319-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0319-z