Abstract
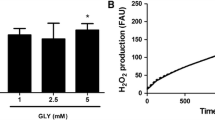
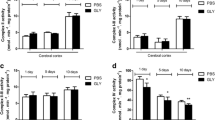
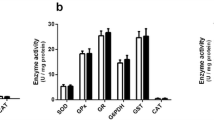
Patients affected by nonketotic hyperglycinemia (NKH) usually present severe neurological symptoms and suffer from acute episodes of intractable seizures with leukoencephalopathy. Although excitotoxicity seems to be involved in the brain damage of NKH, the mechanisms underlying the neuropathology of this disease are not fully established. The objective of the present study was to investigate the in vitro effects of glycine (GLY), that accumulate at high concentrations in the brain of patients affected by this disorder, on important parameters of oxidative stress, such as lipid peroxidation (thiobarbituric acid-reactive substances (TBA-RS) and chemiluminescence) and the most important non-enzymatic antioxidant defense reduced glutathione (GSH) in cerebral cortex from 30-day-old rats. GLY significantly increased TBA-RS and chemiluminescence values, indicating that this metabolite provokes lipid oxidative damage. Furthermore, the addition of high doses of the antioxidants melatonin, trolox (soluble vitamin E) and GSH fully prevented GLY-induced increase of lipid peroxidation, indicating that free radicals were involved in this effect. GLY also decreased GSH brain concentrations, which was totally blocked by melatonin treatment. Finally, GLY significantly reduced sulfhydryl group content from a commercial GSH solution, but did not oxidize reduced cytochrome C. Our data indicate that oxidative stress elicited in vitro by GLY may possibly contribute at least in part to the pathophysiology of the neurological dysfunction in NKH.




Similar content being viewed by others
References
Applegarth DA, Toone JR (2001) Nonketotic hyperglycinemia (glycine encefalophalopathy): laboratory diagnosis. Mol Genet Metab 74:139–146. doi:10.1006/mgme.2001.3224
Behl C, Moosmann B (2002) Oxidative nerve cell death in Alzheimer’s disease and stroke: antioxidants as neuroprotective compounds. Biol Chem 383:521–536. doi:10.1515/BC.2002.053
Berg D, Youdim MB (2006) Role of iron in neurodegenerative disorders. Top Magn Reson Imaging 17:5–17. doi:10.1097/01.rmr.0000245461.90406.ad
Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF (2001) Increased oxidative damage to DNA in a transgenic mouse of Huntington’s disease. J Neurochem 79:1246–1249. doi:10.1046/j.1471-4159.2001.00689.x
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. doi:10.1016/0076-6879(90)86134-H
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi E (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388:261–266. doi:10.1006/abbi.2001.2292
Frazier DM, Summer GK, Chamberlin HR (1978) Hyperglycinuria and hyperglycinemia in two siblings with mild developmental delays. Am J Dis Child 132:777–781
Gonzalez-Flecha B, Llesuy S, Boveris A (1991) Hydroperoxide-initiated chemiluminescence: an assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radic Biol Med 10:93–100. doi:10.1016/0891-5849(91)90002-K
Hall DA, Ringel SP (2004) Adult nonketotic hyperglycynemia crisis presenting as severe chorea and encephalopathy. Mov Disord 19:485–486. doi:10.1002/mds.10681
Halliwell B, Gutteridge JMC (1996) Oxygen radicals and nervous system. Trends Neurosci 8:22–26. doi:10.1016/0166-2236(85)90010-4
Halliwell B, Gutteridge JMC (2007) Measurement of reactive species. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 268–340
Hamosh A, Johnston MV (2001) Nonketotic hyperglycinemia. In: Scriver C, Beaudet A, Valle D et al (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 2065–2078
Hara H, Sukamoto T, Kogure K (1993) Mechanism and pathogenesis of ischemia induced neuronal damage. Prog Neurobiol 40:645–670. doi:10.1016/0301-0082(93)90009-H
Karelson E, Bogdanovic N, Garlind A, Winblad B, Zilmer K, Kullisaar T et al (2001) The cerebrocortical areas in normal brain aging and in the Alzheimer’s disease: noticeable difference in the lipid peroxidation level and in antioxidant defense. Neurochem Res 26:353–361. doi:10.1023/A:1010942929678
Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A (2007) Contribution of endogenous glycine and D-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci 81:740–749. doi:10.1016/j.lfs.2007.07.001
Kono Y, Shigetomi E, Inoue K, Kato F (2007) Facilitation of spontaneous glycine release by anoxia potentiates NMDA receptor current in the hypoglossal motor neurons of the rat. Eur J NeuroSci 25(6):1748–1756. doi:10.1111/j.1460-9568.2007.05426.x
Korman SH, Wexler ID, Gutman A, Rolland MO, Kanno J, Kure S (2006) Treatment from birth of nonketotic hyperglycinemia due to a novel GLDC mutation. Ann Neurol 59(2):411–415. doi:10.1002/ana.20759
Kure S, Tada K, Narisawa K (1997) Nonketotic hyperglycinemia: biochemical, molecular and neurological aspects. Jpn J Hum Genet 42:13–22. doi:10.1007/BF02766917
Lowry OH, Rosebrough NJ, Lewis-Farr A, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L (2006) Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis 10:59–73
McNamara D, Dingledine R (1990) Dual effect of glycine on NMDA-induced neurotoxicity in rat cortical cultures. J Neurosci Res 10(12):3970–3976
Méndez-Álvarez E, Soto-Otero R, Hermida-Aeijeiras A, López-Real AM, Labandeira-García JL (2001) Effects of aluminium and zinc on the oxidative stress caused by 6-hydroxydopamine autoxidation: relevance for the pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1586:155–168
Oda M, Kure S, Sugawara T, Yamaguchi S, Kojima K, Shinka T et al (2007) Direct correlation between ischemic injury and extracellular glycine concentration in mice with genetically altered activities of the glycine cleavage multienzyme system. Stroke 38(7):2157–2164. doi:10.1161/STROKEAHA.106.477026
Patel J, Zinkand WC, Thompson C, Keith R, Salama A (1990) Role of glycine in the N-methyl-D-aspartate-mediated neuronal cytotoxicity. J Neurochem 54(3):849–854. doi:10.1111/j.1471-4159.1990.tb02329.x
Perez-Severiano F, Rios C, Segovia J (2000) Striatal oxidative damage parallels the expression of a neurological phenotype in mice transgenic for the mutation of Huntington’s disease. Brain Res 862:234–237. doi:10.1016/S0006-8993(00)02082-5
Press GA, Barshop BA, Haas RH, Nyhan WL, Glass RF, Hesselink JR (1989) Abnormalities of the brain in nonketotic hyperglycinemia: MR manifestations. AJNR Am J Neuroradiol 10:315–321
Reznick AZ, Packer L (1993) Free radicals and antioxidants in muscular neurological diseases and disorders. In: Poli G, Albano E, Dianzani UM (eds) Free radicals: from basic science to medicine. Birkhauser Verlag, Basel, pp 425–437
Shuman RM, Leech RW, Scout CR (1978) The neuropathology of the nonketotic and ketotic hyperglycinemias: tree cases. Neurology 28:139–146
Spittler A, Reissner CM, Oehler R, Gornikiewicz A, Gruenberger T, Manhart N et al (1999) Immunomodulatory effects of glycine on LPS-treated monocytes: reduced TNF-alpha production and accelerated IL-10 expression. FASEB J 13(3):563–571
Stoy N, Mackay GM, Forrest CM, Christofides J, Egerton M, Stone TW et al (2005) Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem 93:611–623. doi:10.1111/j.1471-4159.2005.03070.x
Tekinalp G, Caskun T, Oran O, Ozalp I, Fiquen G, Erquin H (1995) Nonketotic hyperglycinemia in a newborn infant. Turk J Pediatr 37:57–60
Zhong Z, Jones S, Thurman RG (1996) Glycine minimizes reperfusion injury in a low-flow, reflow liver perfusion model in the rat. Am J Physiol 270:G332–G338
Acknowledgements
This work was supported by grants from CNPq, PRONEX II, FAPERGS, PROPESQ/UFRGS, and FINEP research grant Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leipnitz, G., Solano, A.F., Seminotti, B. et al. Glycine Provokes Lipid Oxidative Damage and Reduces the Antioxidant Defenses in Brain Cortex of Young Rats. Cell Mol Neurobiol 29, 253–261 (2009). https://doi.org/10.1007/s10571-008-9318-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-008-9318-6