Abstract
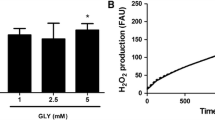
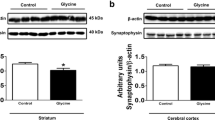
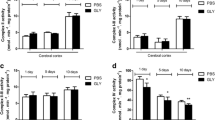
Non-ketotic hyperglycinemia (NKH) is a severe neurological disorder caused by defects in glycine (GLY) catabolism and characterized by a high cerebrospinal fluid/plasma GLY ratio. Treatment is often ineffective and limited to the control of symptoms and detoxification of GLY. In the present work, we investigated the in vivo effects of GLY intracerebroventricular administration on oxidative stress parameters in rat striatum, cerebral cortex, and hippocampus. In vitro effects of GLY were also evaluated in striatum. The effects of bezafibrate (BEZ), a potential neuroprotective agent, on the possible alterations caused by GLY administration were further evaluated. Our in vivo results showed that GLY increased the activities of the antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and glucose-6-phosphate dehydrogenase (G6PDH) in striatum. Furthermore, GLY decreased the concentrations of total glutathione and reduced glutathione (GSH), as well as GSH/oxidized glutathione ratio in vivo in hippocampus. In vitro data also showed that GLY induced lipid peroxidation and decreased GSH in striatum. Regarding the effects of BEZ, we found that GLY-induced increase of GPx, SOD, and GR activities was attenuated or prevented by this compound. However, BEZ did not alter GLY-induced decrease of GSH in hippocampus. We hypothesize that GLY-induced increase of the activities of antioxidant enzymes in striatum occurs as a mechanism to avoid accumulation of reactive oxygen species and consequent oxidative damage. Furthermore, since BEZ prevented GLY-induced alterations, it might be considered as an adjuvant therapy for NKH.





Similar content being viewed by others
References
Hamosh A, Johnston MV (2001) Non-ketotic hyperglycinemia. In: Scriver CR, Beaudet A, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol Editors. 8th edn. McGraw-Hill, New York, pp 2065–2078
Applegarth DA, Toone JR (2001) Nonketotic hyperglycinemia (glycine encephalopathy): laboratory diagnosis. Mol Genet Metab 74(1–2):139–146. https://doi.org/10.1006/mgme.2001.3224
Iqbal M, Prasad M, Mordekar SR (2015) Nonketotic hyperglycinemia case series. J Pediatr Neurosci 10(4):355–358. https://doi.org/10.4103/1817-1745.174445
Hoover-Fong JE, Shah S, Van Hove JL, Applegarth D, Toone J, Hamosh A (2004) Natural history of nonketotic hyperglycinemia in 65 patients. Neurology 63(10):1847–1853
Raghavendra S, Ashalatha R, Thomas SV, Kesavadas C (2007) Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology 49(4):299–305. https://doi.org/10.1007/s00234-006-0189-6
Heindel W, Kugel H, Roth B (1993) Noninvasive detection of increased glycine content by proton MR spectroscopy in the brains of two infants with nonketotic hyperglycinemia. AJNR Am J Neuroradiol 14(3):629–635
Bjoraker KJ, Swanson MA, Coughlin CR 2nd, Christodoulou J, Tan ES, Fergeson M, Dyack S, Ahmad A et al (2016) Neurodevelopmental outcome and treatment efficacy of benzoate and dextromethorphan in siblings with attenuated nonketotic hyperglycinemia. J Pediatr 170:234–239. https://doi.org/10.1016/j.jpeds.2015.12.027
Cusmai R, Martinelli D, Moavero R, Dionisi Vici C, Vigevano F, Castana C, Elia M, Bernabei S et al (2012) Ketogenic diet in early myoclonic encephalopathy due to non ketotic hyperglycinemia. Eur J Paediat Neurol : EJPN : Off J Eur Paediatr Neurol Soc 16(5):509–513. https://doi.org/10.1016/j.ejpn.2011.12.015
Zafrir B, Jain M (2014) Lipid-lowering therapies, glucose control and incident diabetes: evidence, mechanisms and clinical implications. Cardiovasc Drugs Ther 28(4):361–377. https://doi.org/10.1007/s10557-014-6534-9
Corona JC, Duchen MR (2015) PPARgamma and PGC-1alpha as therapeutic targets in Parkinson’s. Neurochem Res 40(2):308–316. https://doi.org/10.1007/s11064-014-1377-0
Procaccio V, Bris C, Chao de la Barca JM, Oca F, Chevrollier A, Amati-Bonneau P, Bonneau D, Reynier P (2014) Perspectives of drug-based neuroprotection targeting mitochondria. Rev Neurol 170(5):390–400. https://doi.org/10.1016/j.neurol.2014.03.005
Johri A, Calingasan NY, Hennessey TM, Sharma A, Yang L, Wille E, Chandra A, Beal MF (2012) Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum Mol Genet 21(5):1124–1137. https://doi.org/10.1093/hmg/ddr541
Kono Y, Shigetomi E, Inoue K, Kato F (2007) Facilitation of spontaneous glycine release by anoxia potentiates NMDA receptor current in the hypoglossal motor neurons of the rat. Eur J Neurosci 25(6):1748–1756. https://doi.org/10.1111/j.1460-9568.2007.05426.x
Moura AP, Grings M, Dos Santos Parmeggiani B, Marcowich GF, Tonin AM, Viegas CM, Zanatta A, Ribeiro CA et al (2013) Glycine intracerebroventricular administration disrupts mitochondrial energy homeostasis in cerebral cortex and striatum of young rats. Neurotox Res 24(4):502–511. https://doi.org/10.1007/s12640-013-9396-1
Moura AP, Parmeggiani B, Grings M, Alvorcem LM, Boldrini RM, Bumbel AP, Motta MM, Seminotti B et al (2015) Intracerebral glycine administration impairs energy and redox homeostasis and induces glial reactivity in cerebral cortex of newborn rats. Mol Neurobiol 53:5864–5875. https://doi.org/10.1007/s12035-015-9493-7
Seminotti B, Knebel LA, Fernandes CG, Amaral AU, da Rosa MS, Eichler P, Leipnitz G, Wajner M (2011) Glycine intrastriatal administration induces lipid and protein oxidative damage and alters the enzymatic antioxidant defenses in rat brain. Life Sci 89 (7–8):276–281. doi:https://doi.org/10.1016/j.lfs.2011.06.013
Moura AP, Grings M, Marcowich GF, Bumbel AP, Parmeggiani B, de Moura Alvorcem L, Wajner M, Leipnitz G (2014) Evidence that glycine induces lipid peroxidation and decreases glutathione concentrations in rat cerebellum. Mol Cell Biochem 395(1–2):125–134. https://doi.org/10.1007/s11010-014-2118-z
Pai YJ, Leung KY, Savery D, Hutchin T, Prunty H, Heales S, Brosnan ME, Brosnan JT et al (2015) Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nat Commun 6:6388. https://doi.org/10.1038/ncomms7388
Grings M, Moura AP, Parmeggiani B, Pletsch JT, Cardoso GMF, August PM, Matte C, Wyse ATS et al (2017) Bezafibrate prevents mitochondrial dysfunction, antioxidant system disturbance, glial reactivity and neuronal damage induced by sulfite administration in striatum of rats: implications for a possible therapeutic strategy for sulfite oxidase deficiency. Biochim Biophys Acta 1863(9):2135–2148. https://doi.org/10.1016/j.bbadis.2017.05.019
Nakajima T, Tanaka N, Kanbe H, Hara A, Kamijo Y, Zhang X, Gonzalez FJ, Aoyama T (2009) Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: a novel peroxisome proliferator-activated receptor alpha-independent mechanism. Mol Pharmacol 75(4):782–792. https://doi.org/10.1124/mol.108.052928
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388(2):261–266. https://doi.org/10.1006/abbi.2001.2292
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Mannervik B, Guthenberg C (1981) Glutathione transferase (human placenta). Methods Enzymol 77:231–235
Leong SF, Clark JB (1984) Regional enzyme development in rat brain. Enzymes associated with glucose utilization. Biochem J 218(1):131–138
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. https://doi.org/10.1385/0-89603-472-0:347
Teare JP, Punchard NA, Powell JJ, Lumb PJ, Mitchell WD, Thompson RP (1993) Automated spectrophotometric method for determining oxidized and reduced glutathione in liver. Clin Chem 39(4):686–689
White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ (2003) Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem 318(2):175–180
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302(2–3):141–145
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Bannister JV (1986) Free radicals in biology and medicine. Barry Halliwell, John M. C. Gutteridge. Q Rev Biol 61 (3):440–441. doi:https://doi.org/10.1086/415130
Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD (2015) Researching glutamate-induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Front Cell Neurosci 9:91. https://doi.org/10.3389/fncel.2015.00091
Goebel DJ, Poosch MS (1999) NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Mol Brain Res 69(2):164–170
Chaturvedi RK, Beal MF (2013) Mitochondria targeted therapeutic approaches in Parkinson’s and Huntington’s diseases. Mol Cell Neurosci 55:101–114. https://doi.org/10.1016/j.mcn.2012.11.011
Valero T (2014) Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 20(35):5507–5509
Schmitt K, Grimm A, Kazmierczak A, Strosznajder JB, Gotz J, Eckert A (2012) Insights into mitochondrial dysfunction: aging, amyloid-beta, and tau-A deleterious trio. Antioxid Redox Signal 16(12):1456–1466. https://doi.org/10.1089/ars.2011.4400
Funding
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX II), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Pró-Reitoria de Pesquisa/Universidade Federal do Rio Grande do Sul (PROPESQ/UFRGS), Financiadora de estudos e projetos (FINEP), Rede Instituto Brasileiro de Neurociência (IBN-Net) # 01.06.0842-00, and Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção (INCT-EN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplementary figure
Effect of intracerebroventricular administration of glycine (GLY, 5 μmol) on total (GS) (a), reduced (GSH) (b) and oxidized glutathione (GSSG) (c) concentrations, GSH/GSSG ratio (d) and γ-glutamate-cysteine ligase (GCL) activity (e) in rat hippocampus 15 min after the administration. Results are presented as mean ± standard deviation for three to five independent experiments (animals). Statistical analysis was performed using Student’s t test for unpaired samples. *P < 0.05, **P < 0.01, compared to controls. (GIF 45 kb)
Rights and permissions
About this article
Cite this article
Parmeggiani, B., Grings, M., da Rosa-Junior, N.T. et al. Bezafibrate Prevents Glycine-Induced Increase of Antioxidant Enzyme Activities in Rat Striatum. Mol Neurobiol 56, 29–38 (2019). https://doi.org/10.1007/s12035-018-1074-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1074-0