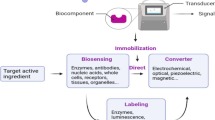
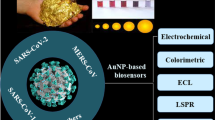
Abstract
In 2019, a worldwide pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged. SARS-CoV-2 is the deadly microorganism responsible for coronavirus disease 2019 (COVID-19), which has caused millions of deaths and irreversible health problems worldwide. To restrict the spread of SARS-CoV-2, accurate detection of COVID-19 is essential for the identification and control of infected cases. Although recent detection technologies such as the real-time polymerase chain reaction delivers an accurate diagnosis of SARS-CoV-2, they require a long processing duration, expensive equipment, and highly skilled personnel. Therefore, a rapid diagnosis with accurate results is indispensable to offer effective disease suppression. Nanotechnology is the backbone of current science and technology developments including nanoparticles (NPs) that can biomimic the corona and develop deep interaction with its proteins because of their identical structures on the nanoscale. Various NPs have been extensively applied in numerous medical applications, including implants, biosensors, drug delivery, and bioimaging. Among them, point-of-care biosensors mediated with gold nanoparticles (GNPSs) have received great attention due to their accurate sensing characteristics, which are widely used in the detection of amino acids, enzymes, DNA, and RNA in samples. GNPS have reconstructed the biomedical application of biosensors because of its outstanding physicochemical characteristics. This review provides an overview of emerging trends in GNP-mediated point-of-care biosensor strategies for diagnosing various mutated forms of human coronaviruses that incorporate different transducers and biomarkers. The review also specifically highlights trends in gold nanobiosensors for coronavirus detection, ranging from the initial COVID-19 outbreak to its subsequent evolution into a pandemic.





© 2021 Elsevier B.V.]

© 2022 Elsevier B.V.]

© 2021 American Chemical Society]


© 2022 Elsevier B.V.]

© 2022 American Chemical Society]

© 2022 Elsevier B.V.]; B Schematic presentation of the SERS-based apt sensor biosensors for the quantitative detection of SARS-CoV-2. Cy3 Raman reporter conjugated on the ending of the aptamer DNAs and 4-mercaptobenzoic acid (internal standard) are bonded on the surface of the gold nanopopcorns substrate [reprinted from Chen et al. with permission from ACS Sens © 2021 American Chemical Society]


© Springer]
Similar content being viewed by others
Data Availability
Data were not used for the research described in the article.
References
Ribeiro, B. V., Cordeiro, T. A. R., Freitas, G. R. O., et al. (2020). Biosensors for the detection of respiratory viruses: A review. Talanta Open, 2, 100007.
Ma, L., Zeng, F., Cong, F., et al. (2019). Development of a SYBR green-based real-time RT-PCR assay for rapid detection of the emerging swine acute diarrhea syndrome coronavirus. Journal of Virological Methods, 265, 66–70.
Zhang, R., & Li, J. (2020). The way to reduce the false negative results of 2019 novel coronavirus nucleic acid detection. Zhonghua Yi Xue Za Zhi, 100, 801–804.
Xie, C., Jiang, L., Huang, G., et al. (2020). Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. International Journal of Infectious Diseases, 93, 264–267.
Zhou, L., Sun, Y., Lan, T., et al. (2019). Retrospective detection and phylogenetic analysis of swine acute diarrhoea syndrome coronavirus in pigs in Southern China. Transboundary and Emerging Diseases, 66, 687–695.
Kang, S., Peng, W., Zhu, Y., et al. (2020). Recent progress in understanding 2019 novel coronavirus (SARS-CoV-2) associated with human respiratory disease: Detection, mechanisms and treatment. International Journal of Antimicrobial Agents, 55, 105950.
Lu, R., Zhao, X., Li, J., et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet, 395, 565–574.
Gao, C., Zhu, L., Jin, C. C., et al. (2021). Prevalence and impact factors of recurrent positive SARS-CoV-2 detection in 599 hospitalized COVID-19 patients. Clinical Microbiology and Infection, 27(785), e781-787.
Krähling, V., Halwe, S., Rohde, C., et al. (2021). Development and characterization of an indirect ELISA to detect SARS-CoV-2 spike protein-specific antibodies. Journal of Immunological Methods, 490, 112958.
WHO. (2023). WHO report on cancer: setting priorities, investing wisely and providing care for all.
Shi, L., Sun, Q., He, J. A., et al. (2015). Development of SPR biosensor for simultaneous detection of multiplex respiratory viruses. Bio-Medical Materials and Engineering, 26, S2207–S2216.
UNCTAD. (2023). UNCTAD annual report 2022. United Nations conference on trade and development, United Nations, Geneva, https://unctad.org/system/files/official-document/osg2023d1_en.pdf assessed 14/03/2024.
McKinsey. (2023). Ten shifts that are transforming organizations and what to do about them. The State of Organizations, https://www.mckinsey.com/~/media/mckinsey/business%20functions/people%20and%20organizational%20performance/our%20insights/the%20state%20of%20organizations%202023/the-state-of-organizations-2023.pdf assessed 14/03/2024.
Hu, F., Qiu, L., Xi, X., et al. (2022). Has COVID-19 changed China’s digital trade?—Implications for health economics. Frontiers in Public Health. https://doi.org/10.3389/fpubh.2022.831549
Qiu, L., Yu, R., Hu, F., et al. (2023). How can China’s medical manufacturing listed firms improve their technological innovation efficiency? An analysis based on a three-stage DEA model and corporate governance configurations. Technological Forecasting and Social Change, 194, 122684.
Zhang, Q., Wang, Y., Bai, R.-T., et al. (2023). X-linked Charcot-Marie-Tooth disease after SARS-CoV-2 vaccination mimicked stroke-like episodes: A case report. World Journal of Clinical Cases, 11, 464.
Franklin, S. M., Crist, M. B., Perkins, K. M., et al. (2022). Outbreak response capacity assessments and improvements among public health department health care-associated infection programs-United States, 2015–2017. Journal of Public Health Management and Practice, 28, 116–125.
Mc Kenna, P., Broadfield, L. A., Willems, A., et al. (2023). Digital health technology used in emergency large-scale vaccination campaigns in low- and middle-income countries: A narrative review for improved pandemic preparedness. Expert Review of Vaccines, 22, 243–255.
Tozzi, A. E., Gesualdo, F., D’Ambrosio, A., et al. (2016). Can digital tools be used for improving immunization programs? Frontiers in Public Health, 4, 36.
Rodriguez-Lonebear, D., Barceló, N. E., Akee, R., et al. (2022). American Indian reservations and COVID-19: Correlates of early infection rates in the pandemic: Erratum. Journal of Public Health Management and Practice, 28, 125.
WHO. (2021). Global strategy on digital health 2020–2025. World Health Organization, Geneva, https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf assessed 14/03/2024.
Chauhan, D. S., Prasad, R., Srivastava, R., et al. (2020). Comprehensive review on current interventions, diagnostics, and nanotechnology perspectives against SARS-CoV-2. Bioconjugate Chemistry, 31, 2021–2045.
Dhar, A., Gupta, S. L., Saini, P., et al. (2023). Nanotechnology-based theranostic and prophylactic approaches against SARS-CoV-2. Immunologic Research, 72, 1–20.
Talebian, S., Wallace, G. G., Schroeder, A., et al. (2020). Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nature Nanotechnology, 15, 618–621.
Weiss, C., Carriere, M., Fusco, L., et al. (2020). Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano, 14, 6383–6406.
Derakhshan, M. A., Amani, A., & Faridi-Majidi, R. (2021). State-of-the-art of nanodiagnostics and nanotherapeutics against SARS-CoV-2. ACS Applied Materials & Interfaces, 13, 14816–14843.
Draz, M. S., Lakshminaraasimulu, N. K., Krishnakumar, S., et al. (2018). Motion-based immunological detection of Zika virus using Pt-nanomotors and a cellphone. ACS Nano, 12, 5709–5718.
Moulick, A., Richtera, L., Milosavljevic, V., et al. (2017). Advanced nanotechnologies in avian influenza: Current status and future trends—a review. Analytica Chimica Acta, 983, 42–53.
Vaculovicova, M., Michalek, P., Krizkova, S., et al. (2017). Nanotechnology-based analytical approaches for detection of viruses. Analytical Methods, 9, 2375–2391.
Wang, H., Sugiarto, S., Li, T., et al. (2016). Advances in nanomaterials and their applications in point of care (POC) devices for the diagnosis of infectious diseases. Biotechnology Advances, 34, 1275–1288.
Zehbe, I., Hacker, G. W., Su, H., et al. (1997). Sensitive in situ hybridization with catalyzed reporter deposition, streptavidin-nanogold, and silver acetate autometallography: Detection of single-copy human papillomavirus. The American Journal of Pathology, 150, 1553.
Caires, A., Mansur, H., Mansur, A., et al. (2019). Gold nanoparticle-carboxymethyl cellulose nanocolloids for detection of human immunodeficiency virus type-1 (HIV-1) using laser light scattering immunoassay. Colloids and Surfaces B: Biointerfaces, 177, 377–388.
Tomichan, R., Sharma, A., Akash, K., et al. (2023). Insight of smart biosensors for COVID-19: A review. Luminescence, 38, 1102–1110.
Mirzadeh-rafie, F., Rahbarizadeh, F., Shoaei, N., et al. (2023). Carbon nanoparticle-based COVID-19 biosensors. Sensors International, 4, 100246.
Oliveira, D. A., Silva, J. V., Flauzino, J. M., et al. (2019). Carbon nanomaterial as platform for electrochemical genosensor: A system for the diagnosis of the hepatitis C in real sample. Journal of Electroanalytical Chemistry, 844, 6–13.
Lao, X., Liu, Y., Li, L., et al. (2023). Plasmon-enhanced FRET biosensor based on Tm3+/Er3+ co-doped core-shell upconversion nanoparticles for ultrasensitive virus detection. Aggregate. https://doi.org/10.1002/agt2.448
Tsang, M.-K., Ye, W., Wang, G., et al. (2016). Ultrasensitive detection of Ebola virus oligonucleotide based on upconversion nanoprobe/nanoporous membrane system. ACS Nano, 10, 598–605.
Adegoke, O., Oyinlola, K., Achadu, O. J., et al. (2023). Blue-emitting SiO2-coated Si-doped ZnSeS quantum dots conjugated aptamer-molecular beacon as an electrochemical and metal-enhanced fluorescence biosensor for SARS-CoV-2 spike protein. Analytica Chimica Acta, 1281, 341926.
Fayyadh, T. K., Ma, F., Qin, C., et al. (2017). Simultaneous detection of multiple viruses in their co-infected cells using multicolour imaging with self-assembled quantum dot probes. Microchimica Acta, 184, 2815–2824.
Hung, L.-Y., Chang, J.-C., Tsai, Y.-C., et al. (2014). Magnetic nanoparticle-based immunoassay for rapid detection of influenza infections by using an integrated microfluidic system. Nanomedicine: Nanotechnology Biology and Medicine, 10, 819–829.
Tarighat, M. A., Ghorghosheh, F. H., Abdi, G. (2022) Fe3O4@SiO2-Ag nanocomposite colorimetric sensor for determination of arginine and ascorbic acid based on synthesized small size AgNPs by cystoseria algae extract. Materials Science and Engineering: B 283, 115855. https://doi.org/10.1016/j.mseb.2022.115855
Yu, L., Adamson, P., Lay Yap, P., et al. (2023). From biowaste to lab-bench: Low-cost magnetic iron oxide nanoparticles for RNA extraction and SARS-CoV-2 diagnostics. Biosensors, 13, 196.
Mokhtarzadeh, A., Eivazzadeh-Keihan, R., Pashazadeh, P., et al. (2017). Nanomaterial-based biosensors for detection of pathogenic virus. TrAC Trends in Analytical Chemistry, 97, 445–457.
Salim, E. T., Fakhri, M. A., Tariq, S. M., et al. (2023). The unclad single-mode fiber-optic sensor simulation for localized surface plasmon resonance sensing based on silver nanoparticles embedded coating. Plasmonics, 19, 1–13.
Hassan, M. E., Yang, Q., Xiao, Z., et al. (2019). Impact of immobilization technology in industrial and pharmaceutical applications. Biotech, 9, 1–16.
Kumar, S., Rathee, G., Bartwal, G., et al. (2023). Biosensors for point-of-care (POC) applications: The flag bearer of the modern medicinal technology to tackle infectious diseases. Point-of-care biosensors for infectious diseases (pp. 69–86). Wiley.
Huang, X., Jain, P. K., El-Sayed, I. H., et al. (2007). Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine, 2, 681.
Oliveira, B. B., Ferreira, D., Fernandes, A. R., et al. (2023). Engineering gold nanoparticles for molecular diagnostics and biosensing. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 15, e1836.
Liu, S., Wei, W., Wang, Y., et al. (2016). Ultrasensitive electrochemical detection of nucleic acid by coupling an autonomous cascade target replication and enzyme/gold nanoparticle-based post-amplification. Biosensors and Bioelectronics, 80, 208–214.
Koo, K. M., Carrascosa, L. G., Shiddiky, M. J., et al. (2016). Amplification-free detection of gene fusions in prostate cancer urinary samples using mrna–gold affinity interactions. Analytical Chemistry, 88, 6781–6788.
Larguinho, M., & Baptista, P. V. (2012). Gold and silver nanoparticles for clinical diagnostics—From genomics to proteomics. Journal of Proteomics, 75, 2811–2823.
Li, Y., Schluesener, H. J., & Xu, S. (2010). Gold nanoparticle-based biosensors. Gold Bulletin, 43, 29–41.
Thaxton, C. S., Georganopoulou, D. G., & Mirkin, C. A. (2006). Gold nanoparticle probes for the detection of nucleic acid targets. Clinica Chimica Acta, 363, 120–126.
Biju, V. (2014). Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chemical Society Reviews, 43, 744–764.
Alafeef, M., Dighe, K., Moitra, P., et al. (2020). Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano, 14, 17028–17045.
Ionescu, R. E. (2023). Updates on the biofunctionalization of gold nanoparticles for the rapid and sensitive multiplatform diagnosis of SARS-CoV-2 virus and its proteins: From computational models to validation in human samples. International Journal of Molecular Sciences, 24, 9249.
Saha, K., Agasti, S. S., Kim, C., et al. (2012). Gold nanoparticles in chemical and biological sensing. Chemical Reviews, 112, 2739–2779.
Shamsipur, M., Emami, M., Farzin, L., et al. (2018). A sandwich-type electrochemical immunosensor based on in situ silver deposition for determination of serum level of HER2 in breast cancer patients. Biosensors and Bioelectronics, 103, 54–61.
Li, S., Zhang, T., Zhu, Z., et al. (2016). Lighting up the gold nanoparticles quenched fluorescence by silver nanoparticles: A separation distance study. RSC Advances, 6, 58566–58572.
Asnaashari, M., Kenari, R. E., Farahmandfar, R., et al. (2018). Fluorescence quenching biosensor for acrylamide detection in food products based on double-stranded DNA and gold nanoparticles. Sensors and Actuators B: Chemical, 265, 339–345.
Lv, X., Zhang, Y., Liu, G., et al. (2017). Aptamer-based fluorescent detection of ochratoxin A by quenching of gold nanoparticles. RSC Advances, 7, 16290–16294.
Wang, W., Kong, T., Zhang, D., et al. (2015). Label-free microRNA detection based on fluorescence quenching of gold nanoparticles with a competitive hybridization. Analytical Chemistry, 87, 10822–10829.
Guo, C., Wang, J., Chen, X., et al. (2018). Construction of a biosensor based on a combination of cytochrome c, graphene, and gold nanoparticles. Sensors, 19, 40.
Roushani, M., & Shahdost-Fard, F. (2016). Fabrication of an electrochemical nanoaptasensor based on AuNPs for ultrasensitive determination of cocaine in serum sample. Materials Science and Engineering: C, 61, 599–607.
Shamsipur, M., Farzin, L., & Tabrizi, M. A. (2016). Ultrasensitive aptamer-based on-off assay for lysozyme using a glassy carbon electrode modified with gold nanoparticles and electrochemically reduced graphene oxide. Microchimica Acta, 183, 2733–2743.
Young, S. L., Kellon, J. E., & Hutchison, J. E. (2016). Small gold nanoparticles interfaced to electrodes through molecular linkers: A platform to enhance electron transfer and increase electrochemically active surface area. Journal of the American Chemical Society, 138, 13975–13984.
Zhu, Y., Sun, S., Yin, X., et al. (2023). Carbon nanotube-gold nanoparticle-based self-powered electrochemical biosensors for highly sensitive and stable detection of myoglobin. ACS Applied Nano Materials, 6, 11085.
Bao, Q., Li, G., Yang, Z., et al. (2023). Electrochemical biosensor based on antibody-modified Au nanoparticles for rapid and sensitive analysis of influenza A virus. Ionics, 29, 2021–2029.
Bharath, G., Naldoni, A., Ramsait, K. H., et al. (2016). Enhanced electrocatalytic activity of gold nanoparticles on hydroxyapatite nanorods for sensitive hydrazine sensors. Journal of Materials Chemistry A, 4, 6385–6394.
Yu, A., Liang, Z., Cho, J., et al. (2003). Nanostructured electrochemical sensor based on dense gold nanoparticle films. Nano Letters, 3, 1203–1207.
Zhang, J., Lahtinen, R. M., Kontturi, K., et al. (2001). Electron transfer reactions at gold nanoparticles. Chemical Communications. https://doi.org/10.1039/b103458h
Zhu, W., Michalsky, R., Metin, O. N., et al. (2013). Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. Journal of the American Chemical Society, 135, 16833–16836.
Aquino, A., Paschoalin, V. M. F., Tessaro, L. L. G., et al. (2022). Updating the use of nano-biosensors as promising devices for the diagnosis of coronavirus family members: A systematic review. Journal of Pharmaceutical and Biomedical Analysis, 211, 114608.
Bu, J., Deng, Z., Liu, H., et al. (2021). Current methods and prospects of coronavirus detection. Talanta, 225, 121977.
Dhar, B. C. (2022). Diagnostic assay and technology advancement for detecting SARS-CoV-2 infections causing the COVID-19 pandemic. Analytical and Bioanalytical Chemistry, 414, 2903–2934.
Drobysh, M., Ramanaviciene, A., Viter, R., et al. (2022). Biosensors for the determination of SARS-CoV-2 virus and diagnosis of COVID-19 infection. International Journal of Molecular Sciences, 23, 666.
Hamidi-Asl, E., Heidari-Khoshkelat, L., Raoof, J. B., et al. (2022). A review on the recent achievements on coronaviruses recognition using electrochemical detection methods. Microchemical Journal, 178, 107322.
Mobed, A., & Shafigh, E. S. (2021). Biosensors promising bio-device for pandemic screening “COVID-19.” Microchemical Journal, 164, 106094.
Mollarasouli, F., Zare-Shehneh, N., & Ghaedi, M. (2022). A review on corona virus disease 2019 (COVID-19): Current progress, clinical features and bioanalytical diagnostic methods. Microchimica Acta, 189, 103.
Bárcena, M., Oostergetel, G. T., Bartelink, W., et al. (2009). Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proceedings of the National Academy of Sciences, 106, 582–587.
Neuman, B. W., Adair, B. D., Yoshioka, C., et al. (2006). Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. Journal of Virology, 80, 7918–7928.
Ren, L.-L., Wang, Y.-M., Wu, Z.-Q., et al. (2020). Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chinese Medical Journal, 133, 1015–1024.
Malik, Y. A. (2020). Properties of coronavirus and SARS-CoV-2. The Malaysian Journal of Pathology, 42, 3–11.
Neuman, B. W., Kiss, G., Kunding, A. H., et al. (2011). A structural analysis of M protein in coronavirus assembly and morphology. Journal of Structural Biology, 174, 11–22.
Chang, C.-K., Sue, S.-C., Yu, T.-H., et al. (2006). Modular organization of SARS coronavirus nucleocapsid protein. Journal of Biomedical Science, 13, 59–72.
de Haan, C. A., & Rottier, P. J. (2005). Molecular interactions in the assembly of coronaviruses. Advances in Virus Research, 64, 165–230.
Peng, Y., Du, N., Lei, Y., et al. (2020). Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design. The EMBO Journal, 39, e105938.
Sanderson, T., Hisner, R., Donovan-Banfield, I. A., et al. (2023). A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes. Nature, 623, 1–3.
Nieto-Torres, J. L., DeDiego, M. L., Álvarez, E., et al. (2011). Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology, 415, 69–82.
Venkatagopalan, P., Daskalova, S. M., Lopez, L. A., et al. (2015). Coronavirus envelope (E) protein remains at the site of assembly. Virology, 478, 75–85.
Kirchdoerfer, R. N., Cottrell, C. A., Wang, N., et al. (2016). Pre-fusion structure of a human coronavirus spike protein. Nature, 531, 118–121.
Song, H. C., Seo, M.-Y., Stadler, K., et al. (2004). Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. Journal of Virology, 78, 10328–10335.
Ou, X., Liu, Y., Lei, X., et al. (2020). Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications, 11, 1620.
Wrapp, D., Wang, N., Corbett, K. S., et al. (2020). Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 367, 1260–1263.
Liu, S., Xiao, G., Chen, Y., et al. (2004). Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: Implications for virus fusogenic mechanism and identification of fusion inhibitors. The Lancet, 363, 938–947.
Li, F., Li, W., Farzan, M., et al. (2005). Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science, 309, 1864–1868.
Lu, G., Hu, Y., Wang, Q., et al. (2013). Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature, 500, 227–231.
Walls, A. C., Park, Y.-J., Tortorici, M. A., et al. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 181(281–292), e286.
Li, G., Fan, Y., Lai, Y., et al. (2020). Coronavirus infections and immune responses. Journal of Medical Virology, 92, 424–432.
Li, X., Geng, M., Peng, Y., et al. (2020). Molecular immune pathogenesis and diagnosis of COVID-19. Journal of Pharmaceutical Analysis, 10, 102–108.
Post, N., Eddy, D., Huntley, C., et al. (2020). Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE, 15, e0244126.
Isho, B., Abe, K. T., Zuo, M., et al. (2020). Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Science Immunology. https://doi.org/10.1126/sciimmunol.abe5511
Pecora, N. D., & Zand, M. S. (2020). Measuring the serologic response to severe acute respiratory syndrome coronavirus 2: Methods and meaning. Clinics in Laboratory Medicine, 40, 603–614.
Rudrapal, M., Khairnar, S. J., & Jadhav, A. G. (2020). Drug repurposing (DR): An emerging approach in drug discovery. Drug repurposing-hypothesis, molecular aspects and therapeutic applications. IntechOpen.
Fang, F. C., Naccache, S. N., & Greninger, A. L. (2020). The laboratory diagnosis of coronavirus disease 2019—Frequently asked questions. Clinical Infectious Diseases, 71, 2996–3001.
WHO. (2020). Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance [BS]. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases (pp. 7–7). Interim guidance.
Azeem, A., Walters, R. W., Cavalieri, S. J., et al. (2023). Reproducibility of cycle threshold values from severe acute respiratory coronavirus virus 2 (SARS-CoV-2) reverse-transcription polymerase chain reaction (RT-PCR) assays. Infection Control & Hospital Epidemiology, 44, 688–689.
Aouissi, H. A., Ababsa, M., & Gaagai, A. (2021). Review of a controversial treatment method in the fight against COVID-19 with the example of Algeria. Bulletin of the National Research Centre, 45, 1–7.
Joung, J., Ladha, A., Saito, M., et al. (2020). Detection of SARS-CoV-2 with SHERLOCK one-pot testing. New England Journal of Medicine, 383, 1492–1494.
Brandsma, E., Verhagen, H. J., van de Laar, T. J., et al. (2021). Rapid, sensitive, and specific severe acute respiratory syndrome coronavirus 2 detection: A multicenter comparison between standard quantitative reverse-transcriptase polymerase chain reaction and CRISPR-based DETECTR. The Journal of Infectious Diseases, 223, 206–213.
Bhimraj, A., Morgan, R. L., Shumaker, A. H., et al. (2020). Infectious diseases society of America guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19). Clinical Infectious Diseases. https://doi.org/10.1093/cid/ciaa478
Infantino, M., Damiani, A., Gobbi, F. L., et al. (2020). Serological assays for SARS-CoV-2 infectious disease: Benefits, limitations and perspectives. The Israel Medical Association Journal, 22, 203–210.
Prince-Guerra, J. L., Almendares, O., Nolen, L. D., et al. (2021). Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites—Pima County, Arizona, November 3–17, 2020. Morbidity and Mortality Weekly Report, 70, 100.
Al Johani, S., & Hajeer, A. H. (2016). MERS-CoV diagnosis: An update. Journal of Infection and Public Health, 9, 216–219.
Hanson, K. E., Caliendo, A. M., Arias, C. A., et al. (2020). Infectious diseases society of America guidelines on the diagnosis of coronavirus disease 2019. Clinical Infectious Diseases. https://doi.org/10.1093/cid/ciaa760
Carter, L. J., Garner, L. V., Smoot, J. W., et al. (2020). Assay techniques and test development for COVID-19 diagnosis. ACS Publications.
Udugama, B., Kadhiresan, P., Kozlowski, H. N., et al. (2020). Diagnosing COVID-19: The disease and tools for detection. ACS Nano, 14, 3822–3835.
Gowri, A., Kumar, N. A., & Anand, B. S. (2021). Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of COVID-19–a minireview. TrAC Trends in Analytical Chemistry, 137, 116205.
Sharma, S., Saini, S., Khangembam, M., et al. (2020). Nanomaterials-based biosensors for COVID-19 detection—A review. IEEE Sensors Journal, 21, 5598–5611.
Murugan, D., Bhatia, H., Sai, V., et al. (2020). P-FAB: A fiber-optic biosensor device for rapid detection of COVID-19. Transactions of the Indian National Academy of Engineering, 5, 211–215.
Miripour, Z. S., Sarrami-Forooshani, R., Sanati, H., et al. (2020). Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosensors and Bioelectronics, 165, 112435.
Michalet, X., Pinaud, F. F., Bentolila, L. A., et al. (2005). Quantum dots for live cells, in vivo imaging, and diagnostics. Science, 307, 538–544.
Srivastava, M., Srivastava, N., Mishra, P., et al. (2021). Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Science of the Total Environment, 754, 142363.
Pishva, P., & Yüce, M. (2021). Nanomaterials to tackle the COVID-19 pandemic. Emergent Materials, 4, 211–229.
Keshavarzi Arshadi, A., Webb, J., Salem, M., et al. (2020). Artificial intelligence for COVID-19 drug discovery and vaccine development. Frontiers in Artificial Intelligence, 3, 65.
Naveed, M., Waseem, M., Aziz, T., et al. (2023). Identification of bacterial strains and development of anmRNA-based vaccine to combat antibiotic resistance in staphylococcus aureus via in vitro and in silico approaches. Biomedicines, 11, 1039.
Naveed, M., Makhdoom, S. I., Ali, U., et al. (2022). Immunoinformatics approach to design multi-epitope-based vaccine against machupo virus taking viral nucleocapsid as a potential candidate. Vaccines, 10, 1732.
Naveed, M., Sheraz, M., Amin, A., et al. (2022). Designing a novel peptide-based multi-epitope vaccine to evoke a robust immune response against pathogenic multidrug-resistant providencia heimbachae. Vaccines, 10, 1300.
Naveed, M., Ali, U., Aziz, T., et al. (2023). A reverse vaccinology approach to design an mRNA-based vaccine to provoke a robust immune response against HIV-1. Acta Biochimica Polonica, 70, 407–418.
Naveed, M., Hassan, J., Aziz, T., et al. (2023). A one-health approach to design an mRNA-based vaccine candidate against the lumpy skin disease virus as an alternative to live-attenuated vaccines. European Review for Medical & Pharmacological Sciences, 27, 6401.
Naveed, M., Mahmood, S., Aziz, T., et al. (2023). Designing a novel chimeric multi-epitope vaccine subunit against Staphylococcus argenteus through artificial intelligence approach integrating pan-genome analysis, in vitro identification, and immunogenicity profiling. Journal of Biomolecular Structure and Dynamics. https://doi.org/10.1080/07391102.2023.2256881
Naveed, M., Ali, U., Aziz, T., et al. (2024). An aedes-anopheles vaccine candidate supplemented with BCG epitopes against the aedes and anopheles genera to overcome hypersensitivity to mosquito bites. Acta Parasitologica. https://doi.org/10.1007/s11686-023-00771-1
Naveed, M., Ali, U., Aziz, T., et al. (2024). Development and immunological evaluation of an mRNA-based vaccine targeting Naegleria fowleri for the treatment of primary amoebic meningoencephalitis. Scientific Reports, 14, 767.
Sarkar, S., Mahato, M., & Gogoi, M. (2023). Nanomaterials for point-of-care biosensors [BS]. Nanobiosensors for point-of-care medical diagnostics (pp. 55–77). Springer.
Zhao, Z., Cui, H., Song, W., et al. (2020). A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv, 2020.2002. 2022.961268.
Tian, B., Gao, F., Fock, J., et al. (2020). Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosensors and Bioelectronics, 165, 112356.
Pinals, R. L., Ledesma, F., Yang, D. W., et al. (2021). Rapid SARS-CoV-2 spike protein detection by carbon nanotube-based near-infrared nanosensors. Nano Letters, 21, 2272–2280.
Li, J., Wu, D., Yu, Y., et al. (2021). Rapid and unamplified identification of COVID-19 with morpholino-modified graphene field-effect transistor nanosensor. Biosensors and Bioelectronics, 183, 113206.
Nguyen, N. H. L., Kim, S., Lindemann, G., et al. (2021). COVID-19 spike protein induced phononic modification in antibody-coupled graphene for viral detection application. ACS Nano, 15, 11743–11752.
Seo, G., Lee, G., Kim, M. J., et al. (2020). Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 14, 5135–5142.
Mahari, S., Roberts, A., Shahdeo, D., et al. (2020). eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2. bioRxiv, 2020.2004.2024.059204.
Qiu, G. G., Gai, Z. B., Tao, Y. L., et al. (2020). Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano, 14, 5268–5277.
Li, Z., Yi, Y., Luo, X., et al. (2020). Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. Journal of Medical Virology, 92, 1518–1524.
Vadlamani, B. S., Uppal, T., Verma, S. C., et al. (2020). Functionalized TiO(2) nanotube-based electrochemical biosensor for rapid detection of SARS-CoV-2. Sensors (Basel), 20, 5871.
Pramanik, A., Gao, Y., Patibandla, S., et al. (2021). The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Advances, 3, 1588–1596.
Huang, J. C., Chang, Y. F., Chen, K. H., et al. (2009). Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosensors and Bioelectronics, 25, 320–325.
Pramanik, A., Gao, Y., Patibandla, S., et al. (2021). Aptamer conjugated gold nanostar-based distance-dependent nanoparticle surface energy transfer spectroscopy for ultrasensitive detection and inactivation of corona virus. The Journal of Physical Chemistry Letters, 12, 2166–2171.
Wang, Z., Zheng, Z., Hu, H., et al. (2020). A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab on a Chip, 20, 4255–4261.
Moitra, P., Alafeef, M., Dighe, K., et al. (2020). Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano, 14, 7617–7627.
Anik, M. I., Hossain, M. K., Hossain, I., et al. (2021). Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Select, 2, 1146–1186.
Anik, M. I., Hossain, M. K., Hossain, I., et al. (2021). Biomedical applications of magnetic nanoparticles [BS]. Magnetic nanoparticle-based hybrid materials (pp. 463–497). Elsevier.
Rubel, M. H., & Hossain, M. K. (2022). Crystal structures and properties of nanomagnetic materials [BS]. Fundamentals of low dimensional magnets (pp. 183–205). CRC Press.
Khizar, S., Al-Dossary, A. A., Zine, N., et al. (2022). Contribution of magnetic particles in molecular diagnosis of human viruses. Talanta, 241, 123243.
Zhong, J., Rosch, E. L., Viereck, T., et al. (2021). Toward rapid and sensitive detection of SARS-CoV-2 with functionalized magnetic nanoparticles. ACS Sensors, 6, 976–984.
Chan, J.F.-W., Kok, K.-H., Zhu, Z., et al. (2020). Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections, 9, 221–236.
Balaban Hanoglu, S., Harmanci, D., Ucar, N., et al. (2023). Recent approaches in magnetic nanoparticle-based biosensors of miRNA detection. Magnetochemistry, 9, 23.
Patel, S., Srivastav, A. K., Gupta, S. K., et al. (2021). Carbon nanotubes for rapid capturing of SARS-COV-2 virus: Revealing a mechanistic aspect of binding based on computational studies. RSC Advances, 11, 5785–5800.
Varghese, R., Salvi, S., Sood, P., et al. (2022). Carbon nanotubes in COVID-19: A critical review and prospects. Colloid and Interface Science Communications, 46, 100544.
Zamzami, M. A., Rabbani, G., Ahmad, A., et al. (2022). Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry, 143, 107982.
Shao, W., Shurin, M. R., Wheeler, S. E., et al. (2021). Rapid detection of SARS-CoV-2 antigens using high-purity semiconducting single-walled carbon nanotube-based field-effect transistors. ACS Applied Materials & Interfaces, 13, 10321–10327.
Liang, Y., Mao, G., Dai, J., et al. (2023). Biofunctionalized semiconductor quantum dots for virus detection. Journal of Semiconductors, 44, 023101.
Ju, B., Zhang, Q., Ge, J., et al. (2020). Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature, 584, 115–119.
Takemura, K., Ganganboina, A. B., Khoris, I. M., et al. (2021). Plasmon nanocomposite-enhanced optical and electrochemical signals for sensitive virus detection. ACS Sensors, 6, 2605–2612.
Lv, P., Zhou, H., Mensah, A., et al. (2018). A highly flexible self-powered biosensor for glucose detection by epitaxial deposition of gold nanoparticles on conductive bacterial cellulose. Chemical Engineering Journal, 351, 177–188.
Maity, D., Murmu, G., Sahoo, S. R., et al. (2023). Metal/metal oxide nanoparticles-based biosensors for detection of infectious diseases. Point-of-care biosensors for infectious diseases (pp. 147–185). Wiley.
Xie, M., Jiang, J., & Chao, J. (2023). DNA-based gold nanoparticle assemblies: From structure constructions to sensing applications. Sensors, 23, 9229.
Banerjee, A., Maity, S., & Mastrangelo, C. H. (2021). Nanostructures for biosensing, with a brief overview on cancer detection, IoT, and the role of machine learning in smart biosensors. Sensors (Basel), 21, 1253.
Gooding, J. J. (2006). Biosensor technology for detecting biological warfare agents: Recent progress and future trends. Analytica Chimica Acta, 559, 137–151.
Zhao, J., Fang, S., Liu, Y., et al. (2020). A lateral flow biosensor based on gold nanoparticles detects four hemorrhagic fever viruses. Analytical Methods, 12, 5613–5620.
Behrouzi, K., & Lin, L. (2022). Gold nanoparticle based plasmonic sensing for the detection of SARS-CoV-2 nucleocapsid proteins. Biosensors and Bioelectronics, 195, 113669.
Farzin, M. A., & Abdoos, H. (2021). A critical review on quantum dots: From synthesis toward applications in electrochemical biosensors for determination of disease-related biomolecules. Talanta, 224, 121828.
Besharati, M., Tabrizi, M. A., Molaabasi, F., et al. (2022). Novel enzyme-based electrochemical and colorimetric biosensors for tetracycline monitoring in milk. Biotechnology and Applied Biochemistry, 69, 41–50.
Holzinger, M., Le Goff, A., & Cosnier, S. (2014). Nanomaterials for biosensing applications: A review. Frontiers in Chemistry, 2, 63.
Jiang, P., Wang, Y., Zhao, L., et al. (2018). Applications of gold nanoparticles in non-optical biosensors. Nanomaterials (Basel), 8, 977.
El-Said, W. A., Al-Bogami, A. S., & Alshitari, W. (2022). Synthesis of gold nanoparticles@reduced porous graphene-modified ITO electrode for spectroelectrochemical detection of SARS-CoV-2 spike protein. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 264, 120237.
Liv, L. (2021). Electrochemical immunosensor platform based on gold-clusters, cysteamine and glutaraldehyde modified electrode for diagnosing COVID-19. Microchemical Journal, 168, 106445.
Zhang, K., Fan, Z., Huang, Y., et al. (2022). A strategy combining 3D-DNA Walker and CRISPR-Cas12a trans-cleavage activity applied to MXene based electrochemiluminescent sensor for SARS-CoV-2 RdRp gene detection. Talanta, 236, 122868.
Yao, B., Zhang, J., Fan, Z., et al. (2021). Rational engineering of the dna walker amplification strategy by using a Au@Ti(3)C(2)@PEI-Ru(dcbpy)(3)(2+) nanocomposite biosensor for detection of the SARS-CoV-2 RdRp gene. ACS Applied Materials & Interfaces, 13, 19816–19824.
Kim, H. E., Schuck, A., Park, H., et al. (2023). Gold nanostructures modified carbon-based electrode enhanced with methylene blue for point-of-care COVID-19 tests using isothermal amplification. Talanta, 265, 124841.
Lambert, C. J., Jayamohan, H., Gale, B. K., et al. (2023). Electrochemical detection of SARS-CoV-2 using immunomagnetic separation and gold nanoparticles on unmodified screen-printed carbon electrodes. Applied Sciences, 13, 10007.
Braz, B. A., Hospinal-Santiani, M., Martins, G., et al. (2023). Gold-binding peptide as a selective layer for electrochemical detection of SARS-CoV-2 antibodies. Talanta, 257, 124348.
Khan, R., Deshpande, A. S., Proteasa, G., et al. (2024). Aptamer-based electrochemical biosensor with S protein binding affinity for COVID-19 detection: integrating computational design with experimental validation of S protein binding affinity. Sensors and Actuators B: Chemical, 399, 134775.
David, H., & Tahir, S. P. (2011). Historical perspectives in diagnostic clinical pathology: Development of the pregnancy test. Journal of Clinical Pathology, 64, 546.
Gootenberg, J. S., Abudayyeh, O. O., Kellner, M. J., et al. (2018). Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science, 360, 439–444.
Zhang, G., Nie, S., Zhang, Z., et al. (2020). Longitudinal change of severe acute respiratory syndrome coronavirus 2 antibodies in patients with coronavirus disease 2019. The Journal of Infectious Diseases, 222, 183–188.
Duan, Y., Wang, S., Zhang, Q., et al. (2021). Nanoparticle approaches against SARS-CoV-2 infection. Current Opinion in Solid State and Materials Science, 25, 100964.
Rosati, M., Agarwal, M., Hu, X., et al. (2021). Control of SARS-CoV-2 infection after spike DNA or spike DNA+protein co-immunization in rhesus macaques. PLOS Pathogens, 17, e1009701.
Maohua, L., Yi, S., Kun, C., et al. (2021). Self-assessment of COVID-19 vaccination efficacy using a lateral flow tests for SARS-CoV-2 S1 protein antibody. medRxiv, 2021.2006.2027.21258591.
Mabrouk, M. T., Chiem, K., Rujas, E., et al. (2021). Lyophilized, thermostable Spike or RBD immunogenic liposomes induce protective immunity against SARS-CoV-2 in mice. Science Advances, 7, 1476.
WHO (2020). Antigen-detection in the diagnosis of Sars-Cov-2 infection using rapid immunoassays. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-usingrapid-immunoassays.
Liu, C., Mao, B., Martinez, V., et al. (2020). A facile assay for rapid detection of COVID-19 antibodies. RSC Advances, 10, 28041–28048.
Wen, T., Huang, C., Shi, F.-J., et al. (2020). Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. The Analyst, 145, 5345–5352.
Huang, C., Wen, T., Shi, F.-J., et al. (2020). Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega, 5, 12550–12556.
Boumar, I., Deliorman, M., Sukumar, P., et al. (2023). Spike-and nucleocapsid-based gold colloid assay toward the development of an adhesive bandage for rapid SARS-CoV-2 immune response detection and screening. Microsystems & Nanoengineering, 9, 82.
Huang, L., Ding, L., Zhou, J., et al. (2021). One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosensors and Bioelectronics, 171, 112685.
Azimi, S., & Docoslis, A. (2022). Recent advances in the use of surface-enhanced Raman scattering for illicit drug detection. Sensors (Basel), 22, 3877.
Arbuz, A., Sultangaziyev, A., Rapikov, A., et al. (2021). How gap distance between gold nanoparticles in dimers and trimers on metallic and non-metallic SERS substrates can impact signal enhancement. Nanoscale Advances, 4, 268–280.
Dinish, U. S., Balasundaram, G., Chang, Y. T., et al. (2014). Actively targeted in vivo multiplex detection of intrinsic cancer biomarkers using biocompatible SERS nanotags. Science and Reports, 4, 4075.
Bistaffa, M. J., Camacho, S. A., Pazin, W. M., et al. (2022). Immunoassay platform with surface-enhanced resonance Raman scattering for detecting trace levels of SARS-CoV-2 spike protein. Talanta, 244, 123381.
Antoine, D., Mohammadi, M., Vitt, M., et al. (2022). Rapid, point-of-care scFv-SERS assay for femtogram level detection of SARS-CoV-2. ACS Sens, 7, 866–873.
Chen, H., Park, S. G., Choi, N., et al. (2021). Sensitive detection of SARS-CoV-2 using a SERS-based aptasensor. ACS Sens, 6, 2378–2385.
Cha, H., Kim, H., Joung, Y., et al. (2022). Surface-enhanced Raman scattering-based immunoassay for severe acute respiratory syndrome coronavirus 2. Biosensors and Bioelectronics, 202, 114008.
Li, Y., Ren, Y., Yi, Z., et al. (2023). Detection of SARS-CoV-2 S protein based on FRET between carbon quantum dots and gold nanoparticles. Heliyon. https://doi.org/10.1016/j.heliyon.2023.e22674
Jamaluddin, N. D., Ibrahim, N., Yusof, N. Y. M., et al. (2023). Optical reflectometric measurement of SARS-CoV-2 (COVID-19) RNA based on cationic cysteamine-capped gold nanoparticles. Optics & Laser Technology, 157, 108763.
Punnoy, P., Siripongpreda, T., Pisitkun, T., et al. (2023). Alternative platform for COVID-19 diagnosis based on AuNP-modified lab-on-paper. The Analyst, 148, 2767–2775.
Tung, Y. T., Chang, C. C., Lin, Y. L., et al. (2016). Development of double-generation gold nanoparticle chip-based dengue virus detection system combining fluorescence turn-on probes. Biosensors and Bioelectronics, 77, 90–98.
Park, T. J., Hyun, M. S., Lee, H. J., et al. (2009). A self-assembled fusion protein-based surface plasmon resonance biosensor for rapid diagnosis of severe acute respiratory syndrome. Talanta, 79, 295–301.
Qiu, X. and Yuan, J. (2006). Temperature control for Pcr thermocyclers based on peltier-effect thermoelectric. In 2005 IEEE engineering in medicine and biology 27th annual conference, IEEE, pp. 7509−7512.
Clavero, C. (2014). Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nature Photonics, 8, 95–103.
Baffou, G., & Quidant, R. (2013). Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser & Photonics Reviews, 7, 171–187.
Nakhleh, M. K., Jeries, R., Gharra, A., et al. (2014). Detecting active pulmonary tuberculosis with a breath test using nanomaterial-based sensors. European Respiratory Journal, 43, 1522–1525.
Shan, B., Broza, Y. Y., Li, W., et al. (2020). Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano, 14, 12125–12132.
Funari, R., Chu, K. Y., & Shen, A. Q. (2020). Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosensors & Bioelectronics, 169, 112578.
Wu, F., Mao, M., Cai, L. Y., et al. (2022). Platinum-decorated gold nanoparticle-based microfluidic chip immunoassay for ultrasensitive colorimetric detection of SARS-CoV-2 nucleocapsid protein. ACS Biomaterials Science & Engineering, 8, 3924–3932.
Qin, J., Tian, X., Liu, S., et al. (2024). Rapid classification of SARS-CoV-2 variant strains using machine learning-based label-free SERS strategy. Talanta, 267, 125080.
Beeram, R., Vepa, K. R., & Soma, V. R. (2023). Recent trends in SERS-based plasmonic sensors for disease diagnostics, biomolecules detection, and machine learning techniques. Biosensors, 13, 328.
Carlomagno, C., Bertazioli, D., Gualerzi, A., et al. (2021). COVID-19 salivary Raman fingerprint: Innovative approach for the detection of current and past SARS-CoV-2 infections. Scientific Reports, 11, 4943.
Ye, J., Yeh, Y.-T., Xue, Y., et al. (2022). Accurate virus identification with interpretable Raman signatures by machine learning. Proceedings of the National Academy of Sciences, 119, e2118836119.
Yang, Y., Peng, Y., Lin, C., et al. (2021). Human ACE2-functionalized gold “virus-trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro letters, 13, 1–13.
Acknowledgements
The authors wish to thank the editors and anonymous reviewers for their valuable comments and suggestions to improve the quality of this article.
Funding
This research did not receive specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
Ethical Approval
Not required for this study.
Consent to Participate
Not required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, A.K., Basavegowda, N., Shirin, S. et al. Emerging Trends of Gold Nanostructures for Point-of-Care Biosensor-Based Detection of COVID-19. Mol Biotechnol (2024). https://doi.org/10.1007/s12033-024-01157-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-024-01157-y