Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative organism of coronavirus disease 2019 (COVID-19) which poses a significant threat to public health worldwide. Though there are certain recommended drugs that can cure COVID-19, their therapeutic efficacy is limited. Therefore, the early and rapid detection without compromising the test accuracy is necessary in order to provide an appropriate treatment for the disease suppression.
Main body
Nanoparticles (NPs) can closely mimic the virus and interact strongly with its proteins due to their morphological similarities. NPs have been widely applied in a variety of medical applications, including biosensing, drug delivery, antimicrobial treatment, and imaging. Recently, NPs-based biosensors have attracted great interest for their biological activities and specific sensing properties, which allows the detection of analytes such as nucleic acids (DNA or RNA), aptamers, and proteins in clinical samples. Further, the advances of nanotechnologies have enabled the development of miniaturized detection systems for point-of-care biosensors, a new strategy for detecting human viral diseases. Among the various NPs, the specific physicochemical properties of gold NPs (AuNPs) are being widely used in the field of clinical diagnostics. As a result, several AuNP-based colorimetric detection methods have been developed.
Short conclusion
The purpose of this review is to provide an overview of the development of AuNPs-based biosensors by virtue of its powerful characteristics as a signal amplifier or enhancer that target pathogenic RNA viruses that provide a reliable and effective strategy for detecting of the existing or newly emerging SARS-CoV-2.
Similar content being viewed by others
1 Background
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has undoubtedly created an emerging disease that is a public health priority worldwide [1,2,3,4,5,6]. This global pandemic has highlighted the urgency of accurate, rapid, and cost-effective diagnostic tests for epidemic understanding and management by monitoring the world’s population [7,8,9,10]. Recently, researchers have focused on developing rapid detection systems because the monitoring and managing of the pandemic are extremely critical. The most widely used current diagnostic method, real-time polymerase chain reaction (RT-PCR) testing, is the gold standard and the most widely available diagnostic tool for SARS-CoV-2 detection [11, 12]. In some countries, it is the only way to declare official results. Other methods are designed on the immunoglobulins detection such as immunoglobulin M (IgM) and/or immunoglobulin G (IgG) [13, 14]. The detection of virus-specific genes by single-stranded DNA probes is of particular interest because of their high sensitivity and specificity compared to antibody- or antigen-based immunological methods for the early diagnosis of viral infections. Many of these molecular and immunological tests have been validated by the Food and Drug Administration (FDA), and commercial kits have been introduced in the field [15].
The development of an efficient, inexpensive, and rapid detection kit will allow infected individuals to perform tests without special knowledge, saving time, and resources. In addition to clinical diagnostic techniques, point-of-care (POC) diagnostic systems can pave the way and start a new era for virus screening [16]. However, considering the specificity- and sensitivity-based shortages and the vulnerabilities in monitoring the spread of the virus, there is a great need to develop integrated intelligent devices based on novel, secure, fast, and accurate diagnostic techniques and implement them on a large scale to curb this outbreak in the world. [15]. Nanoparticles (NPs) are widely used in many medical applications, such as biosensing, drug delivery, imaging, and antimicrobial therapy. Of the nanoparticles, gold nanoparticles (AuNPs) are the most commonly used NPs for viral diagnostic detection due to their unique optical properties, stability, and biocompatibility properties. Over the past 20 years, the unique physicochemical properties of AuNPs have been significantly utilized in clinical diagnostics [17].
The surface plasmon resonance (SPR) phenomenon of AuNPs is responsible for their intense colors, and large absorption and cross-sectional scattering properties [18]. As a result, a number of colorimetric AuNPs-based detection methods have been developed [19,20,21,22]. As in many different technological sections, NPs have demonstrated their appropriateness for biosensing applications. Among noble metal NPs, AuNPs are mostly used for biosensor application [23] due to their biocompatibility, their optical and electronic properties, and their relatively simple production and modification [24]. Like many other technological sections, NPs are suitable for biosensing applications. Of the noble metal NPs, AuNPs are used primarily for biosensor applications [23] due to their biocompatibility, optical and electronic properties, and relatively simple fabrication and modification [24].
This review describes how AuNPs-based biosensors can be used for the detection of SARS-CoV-2 in clinical samples with an aim to develop it as a diagnostic test for COVID-19.
2 Main text
2.1 Overview of SARS-COV-2 diagnosis
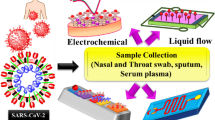
A large-scale diagnosis of SARS-CoV-2 regulates the spread within and across communities and will help to reduce the new coronavirus pandemic [25]. Diagnosis is currently based on a multiplex of criteria, including epidemiology, clinical symptoms, and in vitro diagnostics [14]. Currently, COVID-19 is diagnosed primarily by direct detection of SARS-CoV-2-RNA by nucleic acid amplification assays, most commonly by RT-PCR from the upper respiratory tract [26, 27]. It is complemented by other additional tests, including serological and radiological tests [14, 28].
Various RT-PCR assays are used worldwide; various assays amplify and detect different regions of the SARS-CoV-2 genome. Some target two or more genes, including the nucleocapsid (N), envelope (E), and spike (S) genes, as well as the regions of the first open reading frame, including the RNA-dependent RNA polymerase (RdRp) gene [29]. Although the E assay is specific for all SARS-CoV-associated viruses, the RdRp test only detects COVID-19 virus, but for laboratory confirmation, it is recommended that E be followed by RdRp. However, in areas where SARS-CoV-2 is widely spread, positive RT-PCR test result needs detection of at least one target gene, with priority to the E gene being more sensitive [29]. Other, less common types of nucleic acid amplification assays include isothermal amplification, clustered regularly interspaced short palindromic repeats (CRISPR)-based assays, and next-generation sequencing [30,31,32]. Rapid RT-PCR assays provide similar performance comparably to standard laboratory-based nucleic acid amplification assays, but rapid isothermal assays are less sensitive [33, 34].
Serological tests detect antigens and antibodies directed against the coronavirus. SARS-CoV-2 belongs to the same β-coronavirus family that caused the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome respiratory syndrome (MERS) epidemics, and it is expected to have a similar antibody production process [35] where there is a delay of 14–28 days after the onset of the disease until the antibodies appear in the serum of patients [36]. In setting where access to nucleic acid amplification tests is limited or expensive, antigen tests can be used as an initial test, but antigen tests are less sensitive than nucleic acid amplification tests, and negative antigen tests should be confirmed by additional tests. Assays that detect SARS-CoV-2 antigen can be performed quickly and at the point of care and allowing faster access to results than some nucleic acid amplification assays. Antigen assays are generally less sensitive than nucleic acid amplification assays [37,38,39]. Nevertheless, they can be useful when nucleic acid amplification tests are not available or where nucleic acid amplification tests turnaround times are too long to be clinically useful, provided that clinicians are aware of the possibility of false negative results and interpret the results according to the pretest probability of COVID-19.
For more accurate results, a combined IgG and IgM antibody test is recommended [35]. Serologic tests detect antibodies to SARS-CoV-2 in the blood, and those that have been adequately validated can help identify patients who previously had SARS-CoV-2 infection as well as patients with current infection who have had symptoms for three to four weeks. Serological tests have very limited diagnostic benefit in acute cases because they are less likely to be reactive in the first days to weeks of infection [27, 40]. Because the sensitivity of the test is uncertain after 5 weeks, the accuracy of the test is optimized by confirming the serological test 3–4 weeks after the onset of symptoms [41]. In the reported study, the mean time to seroconversion was 12 days, while positive RT-PCR is observed 5–6 days after the onset of symptoms, suggesting that antibody testing is still worse than RT-PCR in COVID-19 diagnosis but more likely used when RT-PCR is not available or accessible [42].
2.2 Nanotechnology-based biosensors
Use of biosensors is the most recent and advanced tool for diagnostics. The nanotechnology-based biosensors consist of a receptor (receive samples), a transducer (to processes sample), and a reading system to indicate result [43]. The successes of biosensors are due to their high sensitivity and specificity, whereas the use of nanomaterials makes them able to react to biomarkers at low potential [44]. Continuous monitoring of patient health status and a rapid decision making are two vital steps for better health care services during pandemic situation like COVID-19 [45].
The recent developments in nanotechnology made it possible to use different nanomaterials as electrodes in nanosensors resulting in production of nanobiosensors. Various nanomaterials such as nanofilms, nanowires, nanotubes, and nanotubes of different materials are either under use or development stages. Some of these nanomaterial-based biosensors are described as follows:
2.2.1 Quantum dots-based biosensors
Quantum dot (QD)-based nanoparticles range from 2 to 10 nm in size with unique electrical and optical properties and a very versatile nature [46]. These particles are made of cadmium selenide or indium phosphate core, an outer shell of zinc sulfide, and an upper most organic coating to give a hydrophilic property to the particle for its better connectivity with biomolecules (proteins and oligonucleotides) [47]. Due to broad spectrum property of QD-based nanoparticles from red to blue light make them suitable to be used in medical imaging, labeling, and sensing biosensors specially to differentiate between normal and tumor cells [48]. Some researchers used magnetic QD and magnetic nanoparticles for antibiotic detection. Meng and co-workers developed an innovative method to identify five antibiotic residues (quinolone) in various food items [49]. Li et al. combined QDs with Eu3+ for detecting tetracycline in environmental and biological samples [50].
2.2.2 Carbon nanoparticle- and nanotube-based biosensors
Use of carbon nanoparticles and nanotubes (CNT) in biosensors is introduced due to their low cost [51], well-organized structure, and having combination of many unique properties including magnetic, electric, and chemical ones [52]. CNT are important due to their capacity to pass biological barriers easily and can penetrate individual cells easily [53]. Carbon nanoparticle-based and CNT-based biosensors are used for various diagnostic methods including enzymes, antibody, polypeptides, DNA, RNA, and aptamer. Lubbers and Oppitz developed first fiber optics-based biosensor known as ‘optode’ to measure CO2, O2, and alcohol in cells and tissues [54]. Nanobiosensors based on CNT are used to detect DNA sequences related to specific disease condition. These biosensors can be easily inserted in body without causing inflammation and can eliminate need of blood drawing or repeated sample collection.
2.2.3 Metal nanoparticle-based biosensors
AuNPs are commonly used for the development of electrochemical and optical biosensors [55]. Wei et al. constructed AuNP biosensors with boron-doped diamond electrodes to detect organophosphate pesticide, which is used in agriculture. AuNPs on carbon spheres are used as colorimetric biosensors for detection of oligonucleotide or DNA [56]. AuNP-based biosensors are sensitive to 10–11 mol/L. Due to its high sensitivity, Su et al. [57] used AuNPs-based biosensors for Bacillus anthracis using QCM (quartz crystal microbalance). They immobilized thiolated DNA probe on AuNP having a complementary DNA sequence with extended sequence. After introduction, the signals amplified with DNA-conjugated AuNP lower the detection limits of bacteria by 3.5 × 102 CFU/ml and reduce the use of carcinogenic reagents for detection [57]. The recent researches on nanomaterial-based biosensors are still going on with many modifications, and area of diagnostics is increasing as in case of COVID-19. AuNP-based biosensors are in use and development for the diagnostic use in detecting and monitoring COVID-19 disease.
2.3 Gold nanoparticle-based biosensors
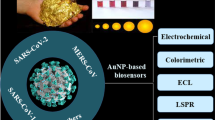
AuNPs have the characteristics of being inert, biocompatible, and non-toxic, as well as being easy to synthesize, changing their surface functionality, and being adjustable in size and shape. Therefore, it has stimulated interest in interfacing biorecognition systems with signal transduction and as determining agents in the design and implementation of functional biosensing devices [58, 59]. Biosensors that generate signals in proportion to the amount of analyte to be analyzed have many uses. These are mainly viral diagnosis during the COVID-19 pandemic period, other clinical diagnoses, monitoring of clinical processes, bioreactors, drug production and release. Achieving results in a short time and ease of application are the most important advantages of biosensors [60] (Fig. 1). AuNPs-based biosensors can be classified as optical biosensors, electrochemical biosensors, and piezoelectric biosensors. Below is an introduction to various types of GNP-based biosensors. The focus is on the effects of AuNPs on the biosensing process and the mechanism of improving analytical performances (Table 1).
2.3.1 Development of gold nanoparticle-based biosensors
With the development of AuNPs-based biosensors, the properties of AuNPs have been used more extensively in the diagnostic process. The most critical point during development and design is that system behaviors are predictable and repeatable. Nano-PCR, an important tool for the diagnosis of viral diseases, is highly sensitive and dependent on the effects of AuNPs. AuNPs can be easily functionalized according to their shape, size, and aggregation properties. Hamdy et al. [61] reported that a new AuNPs biosensor was developed, using oligonucleotides recognizing certain genes of FMDV by developing a specific technique to diagnose the foot-and-mouth disease virus [61, 62]. The development of AuNPs-based biosensors based on their interaction with different substrates has also been very useful in colorimetric studies. Biosensors need to be modified and improved for long-term reliability, extended shelf life, and customization of operation and design [63].
Particularly for SARS-CoV-2, which is the COVID-19 virus, an economical and rapid test methodology has been developed to combat virus-induced pandemics by detecting nucleocapsid (N) proteins and for POC (point-of-care) applications. The methodology was combined with the localized plasmon resonance (LPR) principle of collecting antigen-coated AuNPs, and quantitative concentration values were measured with optical spectrometers. In this way, the presence of viruses can be detected by color change [32].
As mentioned above, AuNPs have excellent photoelectric properties and also resist DNAzyme transfection and nuclease degradation. For this reason, DNAzyme–AuNPs complexes have been the focus of attention in biosensing and bioimaging. It is used as a probe to increase and maintain catalytic efficiency, detect the molecular recognition signal, and convert it into a detectable physicochemical signal. In the future, these probes are very important for the detection of targets [62]. The development of versatile biosensors requires modulation and coordinated operation of different components. Although efficient synergy is not always possible, building a multifunctional biosensor will effectively reduce costs. In a related study, a three-dimensional cluster of AuNPs/ferrocene/liposome was fabricated to fabricate the electrochemical biosensor. In this way, electrochemical analysis of lipopolysaccharides (LPS) has shown great potential in terms of significant versatility and economic cost [63]. Another developed biosensor system is on viruses. They developed a biosensor based on displacement amplification using magnetic beads and enzymes in order to detect the pathogen in a shorter time and with less cost for the hemorrhagic fever virus, which is the pathogen of viral hemorrhagic fever disease (VHF) [54]. Hepatitis B virus (HBV), which causes cirrhosis, liver cancer, and many health problems if not managed properly, needs a precise diagnostic technique. For this purpose, various biosensors have been developed and allow timely intervention against this virus [64, 65]. Ultra-sensitivity and small size are important requirements in the development of new biosensors. Various microfluidic technologies integrated with technologies such as nanotubes, nanoparticles, detectors, and biosensors will be able to detect biological and chemical active substances faster, more precisely, at a lower cost, and more sensitively in many areas [66].
In another study with nanomaterials, oxidized thin films and AuNPs were combined and the capacitance values between DNA immobilization and hybridization were measured by means of an electrochemical DNA biosensor, and it was seen that AuNPs and thin films were successful in DNA detection [67].
2.3.2 Evolution of gold nanoparticle-based biosensors
The optical, chemical, and physical properties of AuNPs make them strong candidates for designing new biosensors and imaging methods. The development of AuNPs-based biosensors offers cost-effective and rapid strategies in unexpected conditions such as the COVID-19 pandemic process. The promising analytical behavior and unique and superior properties of AuNPs have shown advantageous performances for three main types of biosensors. Most diagnoses in the COVID-19 pandemic continue to be made by PCR, a costly and lengthy process. It is clear that virus-induced pandemics can be overcome with the development of AuNPs-based biosensors. Biosensors need agents with properties such as reliability, selectivity, and sensitivity in order to perform more analytically. Although there have been many developments in AuNPs-based biosensors, a widespread practical use is currently not available. Some challenges must be overcome in order for these biosensors to reach their full potential. But the generation of long-term AuNPs-based biosensors is promising. The use of AuNPs in biosensors should be encouraged, as their optical and electrical properties greatly affect the biosensors. As exemplified above, combining it with other nanomaterials and creating a synergistic effect remain interesting in the diagnostic process. In addition to the discovery of these new structure processes, preventing the adsorption process, obtaining reproducible results, and shortening the analysis time will improve the analytical performance of biosensors [68,69,70,71].
3 Gold nanoparticle-based biosensors in COVID-19 diagnosis
To confirm SARS-CoV-2 infection, diagnostic procedures that identify viral nucleic acids, viral antigens, or serological testing are necessary. Rapid antigenic and rapid antibody tests have shorter execution times (15–30 min), a cheaper cost, and a simpler method that really does not need highly skilled staff [72]. The majority of COVID-19 immunoenzymatic serological tests are based on the indirect enzyme-linked immunosorbent assay principle (ELISA) [73]. This method allows researchers to acquire extremely precise and sensitive data in a short period of time (between 1 and 3 h). There are a variety of alternative methods for diagnosing COVID-19 infection, such as viral culture and electron microscopy, NGS, clinical investigations and imaging techniques (CT Scan), biosensor COVID-19 testing techniques, loop-mediated isothermal amplification (LAMP), CRISPR/Cas-based COVID-19 testing methods, and Digital PCR COVID-19 testing methods, but the effectiveness and specificity of these methods are still questionable [74].
The fast immunochromatographic test strip, also known as lateral flow immunoassay (LFIA), was created as a consequence of the confluence of numerous threads dating back to the era [75]. The notion of fast diagnostic tests based on bodily fluids, on the other hand, dates back far longer. The basic concepts of lateral flow technology were developed throughout the early 1980s and found throughout the latter years of that era, with businesses such as Becton Dickinson & Co., Unilever, and Carter Wallace submitting numerous important patents on this technological format [76]. The human pregnancy test, which reflected a continuing historical interest in urine testing for medical diagnostic purposes, was the dominant material driving the initial stages of solid-phase, rapid test technology [77]. Improvements in antibody production methods and substantial advancements in knowledge of the biology and detection of human chorionic gonadotropin (hCG), primarily due to Vaitukaitis and co-workers' work, propelled this specific diagnostic application forward in the 1970s. However, a variety of additional enabling technologies were necessary to properly create the lateral flow test platform [78].
3.1 LFIA
Assays are traditionally made up of a range of components, each of which serves one or more roles. The components are layered on top of each other and adhered to a base card with pressure-sensitive epoxy. The assay consists of several zones including sample pad, conjugate pad, test line, etc. The sample is placed on the sample pad initially to make it compatible with the subsequent zones. This sample runs in the conjugate pad, which is the following zone. To produce a color response, the conjugate pad includes a small number of proteins, either antibodies or antigens, complexes with any enzyme or nanoparticle [79]. These proteins bind to the biological component in the sample that is present. These conjugate protein and biological sample complexes move to a new zone known as the test line [80]. This conjugate compound is caught by particular proteins in the test line. The conjugate enzyme is activated by interaction at the test line, resulting in a color response. To avoid cross-reactivity, the leftover proteins or biological components will be sent to the absorbent.
Improving test sensitivity and selectivity is critical for effective therapy. The existence of viral material and the measurement of the proportion of infection produced in the body are both significant. Scientists are working on new ways to improve the diagnostic selectivity and sensitivity. In the realm of nucleic acid detection, gold nanoparticles (AuNPs) have emerged as league nanomaterials [81]. The antigen–antibody complex is extremely simple during LFI, whereas in the case of AuNPs, antigen–antibody combination creates an aggregation with AuNPs. The AuNPs bind to multiple anti-SARS-CoV-2 antibodies and form a complex [82]. This complex when interacts with the SARS-CoV-2 antigens produces highly intensive color, which gives accurate results in colorimetry with good selectivity and specificity [83].
The gold nanoparticle diagnostic kit makes antigen identification from a swab simple. The Au anti-SARS-2 antibodies are included in the conjugate pad [84]. If the sample pad contains SARS-2 antigens, the antigens bind to the Au anti-SARS-2 antibodies throughout the run, forming antigen–antibody complexes. This binding is very complex because one gold nanoparticle binds to more than two anti-SARS-2 antibodies, indirectly causing multiple antigens to bind to one gold nanoparticle, resulting in a vibrant color test line. This vibrant color aids in accurate detection. Few Au anti-SARS-2 antibodies remain unbonded in the conjugate pad. The complexes of Au anti-SARS-2 antibodies and SARS-2 antigens, as well as unbound Au anti-SARS-2 antibodies, will rush to the test line [85]. The Au anti-SARS-2 antibodies–SARS-2 antigens complexes bind to the anti-SARS-2 antibodies at the test line, leaving the unbonded Au anti-SARS-2 antibodies unbound. The complexity of Au anti-SARS-2 antibodies–SARS-2 antigens–anti-SARS-2 antibodies rises to a next level, resulting in a very intense color generation. Secondary antibodies in the control line simply attach to the unbound Au anti-SARS-2 antibodies and produce color. These diagnostic kits are extremely easy to identify. The presence of SARS-2 antigens is shown by the color of the test line. The presence of Au anti-SARS-2 antibodies is indicated by the color on the control line, indicating that the kit is functioning properly [86]. The absence of color at the control line indicates that the kit is not in functioning order. The presence of the antigen (positive result) is shown by the color of the test and control lines, whereas the absence of the antigen (negative result) is indicated by the color only at the control line (Fig. 2).
The gold nanoparticle diagnostic kit makes antibody identification simple. The Au SARS-COV-2 antigens and Au rabbit IgG conjugate are included in the conjugate pad. If the sample pad contains SARS-COV-2 antibodies, the antibodies bind to the Au SARS-COV-2 antigens throughout the run, forming antigen–antibody complexes. This binding is very complex because one gold nanoparticle binds to more than two SARS-COV-2 antigens, indirectly causing multiple antibodies to bind to one gold nanoparticle, resulting in extreme color test line [87]. This extreme color aids in accurate detection. Au Rabbit IgG Conjugate and few Au SARS-COV-2 antigens remain unbonded in the conjugate pad. The complexes of Au anti-SARS-2 antigens and SARS-2 antibodies, as well as unbound Au Rabbit IgG Conjugate and few Au SARS-COV-2 antigens, will rush to the test line. For detection of the antibodies, there are two test lines (M line and G line) [88]. The Au anti-SARS-2 antigens–SARS-2 IgM antibodies complexes bind to the Anti-Human IgM antibodies at the M test line, leaving the unbonded Au anti-SARS-2 antibodies and Au anti-SARS-2 antigens–SARS-2 IgG antibodies unbound [89]. The Au anti-SARS-2 antigens–SARS-2 IgG antibodies complexes bind to the Anti-Human IgG antibodies at the G test line. The complexity of Au anti-SARS-2 antigens–SARS-2 antibodies–Anti-Human antibodies rises to a next level, resulting in a very intense color generation. Anti-rabbit IgG antibodies in the control line simply bind to the unbound Au rabbit IgG Conjugate and produce color. The presence of SARS-2 antibodies is shown by the color of the test line. The presence of Au rabbit IgG Conjugate is indicated by the color on the control line, indicating that the kit is functioning properly. The presence of the antibodies (positive result) is shown by the color of the test and control lines [90].
4 Challenges and perspectives
At present, the impact of SARS-COV-2 virus on the immune system has been explored thoroughly. The well-implemented nanosystems have made the treatment procedure easier through the development of diagnostic tools [91]. However, the major challenge faced in implementation of diagnostic procedures globally is the variations observed in the outcomes with respect to region, race, gender, and age. Thorough insights to documented cases of COVID-19 clearly indicate that the severity, as well as symptoms, of infection presents itself as a unique scenario in every patient [92]. Nanotechnology can speed the diagnostic processes in mass population. However, for above reasons and the detection of severity of organ or systemic damage, a more personalized approach is required for effective patient treatment. To a large extent, this can be achieved through integration of nanotechnology techniques with that of artificial intelligence with the help of smart bedside monitoring systems. Through this integration, we can not only facilitate the diagnosis of infectious agents but also design evidence-based planning strategies for a more personalized treatment regimen [93].
On a molecular level, a major challenge is to develop biosensing devices with improved sensitivity to detect low titer of viruses in the samples. Considering the multiple processing steps involved during the use of biosensing devices like RNA extraction, cDNA amplification, and signal transduction, the sensitivity of the procedure can be easily compromised [94]. This necessitates the need for the use of ultra-pure reagents during development of biosensing devices and well-qualified and trained individuals for carrying out the diagnosis. Additionally, compliance with these factors alone is obscured in the absence of centralized biosafety laboratories required for handling biohazardous virus samples. Overall, these steps enhance the economic burden on the centralized healthcare systems as well as the community. To overcome these shortcomings, CRISPR-based devices used in ‘Point of Care’ diagnosis are suitable alternatives. However, they require more congruous approach and primer designs for every target nucleic acids [95].
Another technique for COVID-19 diagnosis based on serological antigen detection and antibody response is the preferred and standard diagnostic procedure for infectious diseases. However, these protein and enzyme-based techniques are highly sensitive to external factors and testing reagents. Besides, the cross-reactivity of these proteins with other coronaviruses can lead to false-positive results. Specifically, it is reported that the S protein of SARS-CoV frequently binds to the SARS-CoV-2 antibody leading to false-positive outcome. Also, the challenges of screening mass population during epidemics are enhanced with reliance on serological tests because they only detect the antibodies after it reaches a suitable titer after incubation.
5 Conclusions
Epidemic outbreaks are a challenging scenario for the healthcare systems. We have witnessed a profound impact of COVID-19 pandemic on the economic and social aspects of the society. As summarized in this article, the use of nanotechnology-aided diagnostic approaches can help in rapid detection of SARS-COV-2 virus. In turn, it may allow early treatment of infection and gradually eradicate the virus by implementing thorough screening programs. In order to accomplish this goal, it is necessary to develop a reliable and universal diagnostic method that is affordable and highly sensitive and allows screening of mass population. Several advances with respect to diagnostic approaches using biomarkers, biosensors, and functional detection systems have been suggested in the literature. However, even the most suitable techniques are accompanied with several challenges. We are required to overcome these challenges soon to avoid the intensity of infectious diseases and in future prevent them from becoming a global epidemic. So far, the efficacy, stability as well as safety of nanotechnology-based diagnostic approaches as compared to other techniques hold promise in accomplishment of our goals to combat SARS-COV-2 infection.
Availability of data and materials
Not applicable.
Abbreviations
- IgG:
-
Immunoglobulin M
- IgG:
-
Immunoglobulin G
- FDA:
-
Food and Drug Administration
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus diseases 2019
- SARS:
-
Severe acute respiratory syndrome
- MERS:
-
Middle East respiratory syndrome respiratory syndrome
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- POC:
-
Point-of-care
- NPs:
-
Nanoparticles
- AuNPs:
-
Gold nanoparticles
- SPR:
-
Surface plasmon resonance
- RdRp:
-
RNA-dependent RNA polymerase
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- QD:
-
Quantum dot
- CNT:
-
Carbon nanotubes
- QCM:
-
Quartz crystal microbalance
- ELISA:
-
Enzyme-linked immunosorbent assay principle
- LFA:
-
Lateral flow immunoassay
References
Priyanka CO, Singh I (2020) Diagnosis of SARS-CoV-2: a review on the current scenario and future outlook. Acta Virol 64:396–408. https://doi.org/10.4149/av_2020_402
Aouissi HA (2021) Algeria’s preparedness for Omicron variant and for the fourth wave of COVID-19. Glob Health Med 3:413–414. https://doi.org/10.35772/ghm.2021.01117
Lounis M, Rais MA, Bencherit D, Aouissi HA, Oudjedi A, Klugarová J, Pokorná A, Klugar M, Riad A (2022) Side effects of COVID-19 inactivated virus vs. adenoviral vector vaccines: experience of Algerian healthcare workers. Front Public Health 10:896343. https://doi.org/10.3389/fpubh.2022.896343
Habas K, Nganwuchu C, Shahzad F, Gopalan R, Haque M, Rahman S, Majumder AA, Nasim T (2020) Resolution of coronavirus disease 2019 (COVID-19). Expert Rev Anti Infect Ther 18:1201–1211. https://doi.org/10.1080/14787210.2020.1797487
Rudrapal M, Khairnar SJ, Borse LB, Jadhav AG (2020) Coronavirus disease-2019 (COVID-19): an updated review. Drug Res 70:389–400. https://doi.org/10.1055/a-1217-2397
Rudrapal M, Celik I, Chinnam S, Ansari MA, Khan J, Alghamdi S, Almehmadi M, Zothantluanga JH, Khairnar SJ (2022) Phytocompounds as potential inhibitors of SARS-CoV-2 Mpro and PLpro through computational studies. Saudi J Biol Sci 29:3456–3465. https://doi.org/10.1016/j.sjbs.2022.02.028
Beduk T, Beduk D, de Oliveira Filho JI, Zihnioglu F, Cicek C, Sertoz R, Arda B, Goksel T, Turhan K, Salama KN, Timur S (2021) Rapid point-of-care COVID-19 diagnosis with a gold-nanoarchitecture-assisted laser-scribed graphene biosensor. Anal Chem 93:8585–8594. https://doi.org/10.1021/acs.analchem.1c01444
Leveau CM, Aouissi HA, Kebaili FK (2022) Spatial diffusion of COVID-19 in Algeria during the third wave. GeoJournal. https://doi.org/10.1007/s10708-022-10608-5
Aouissi HA, Belhaouchet I (2021) What about rheumatic diseases and COVID-19? New Microbes New Infect 41:100846. https://doi.org/10.1016/j.nmni.2021.100846
Fenollar F, Bouam A, Ballouche M, Fuster L, Prudent E, Colson P, Tissot-Dupont H, Million M, Drancourt M, Raoult D, Fournier PE (2021) Evaluation of the Panbio COVID-19 rapid antigen detection test device for the screening of patients with COVID-19. J Clin Microbiol 59:e02589-e2620. https://doi.org/10.1128/JCM.02589-20
Feng W, Newbigging AM, Le C, Pang B, Peng H, Cao Y, Wu J, Abbas G, Song J, Wang DB, Cui M (2021) Molecular diagnosis of COVID-19: challenges and research needs. Anal Chem 92:10196–10209. https://doi.org/10.1021/acs.analchem.0c02060
Ter-Ovanesyan D, Gilboa T, Lazarovits R, Rosenthal A, Yu X, Li JZ, Church GM, Walt DR (2021) Ultrasensitive measurement of both SARS-CoV-2 RNA and antibodies from saliva. Anal Chem 93:5365–5370. https://doi.org/10.1021/acs.analchem.1c00515
Clemente A, Alba-Patiño A, Santopolo G, Rojo-Molinero E, Oliver A, Borges M, Aranda M, Del Castillo A, De La Rica R (2021) Immunodetection of lung IgG and IgM antibodies against SARS-CoV-2 via enzymatic liquefaction of respiratory samples from COVID-19 patients. Anal Chem 93:5259–5266. https://doi.org/10.1021/acs.analchem.1c00251
Priyanka OP, Choudhary SI (2020) Diagnosis of SARS-CoV-2: a review on the current scenario and future outlook. Acta Virol 64:396–408. https://doi.org/10.4149/av_2020_402
Sharafeldin M, Davis JJ (2020) Point of care sensors for infectious pathogens. Anal Chem 93:184–197. https://doi.org/10.1021/acs.analchem.0c04677
Cordeiro M, Ferreira Carlos F, Pedrosa P, Lopez A, Baptista PV (2016) Gold nanoparticles for diagnostics: advances towards points of care. Diagnostics 6:43. https://doi.org/10.3390/diagnostics6040043
Huang X, Jain PK, El-Sayed IH, El-Sayed MA (2007) Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2:681–693. https://doi.org/10.2217/17435889.2.5.681
Liu S, Wei W, Wang Y, Fang L, Wang L, Li F (2016) Ultrasensitive electrochemical detection of nucleic acid by coupling an autonomous cascade target replication and enzyme/gold nanoparticle-based post-amplification. Biosens Bioelectron 80:208–214. https://doi.org/10.1016/j.bios.2016.01.067
Koo KM, Carrascosa LG, Shiddiky MJ, Trau M (2016) Amplification-free detection of gene fusions in prostate cancer urinary samples using mRNA-gold affinity interactions. Anal Chem 88:6781–6788. https://doi.org/10.1021/acs.analchem.6b01182
Thaxton CS, Georganopoulou DG, Mirkin CA (2006) Gold nanoparticle probes for the detection of nucleic acid targets. Clin Chim Acta 363:120–126. https://doi.org/10.1016/j.cccn.2005.05.042
Larguinho M, Baptista PV (2012) Gold and silver nanoparticles for clinical diagnostics: from genomics to proteomics. J Proteomics 75:2811–2823. https://doi.org/10.1016/j.jprot.2011.11.007
Li Y, Schluesener HJ, Xu S (2010) Gold nanoparticle-based biosensors. Gold Bull 43:29–41. https://doi.org/10.1007/BF03214964
Biju V (2014) Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev 43:744–764. https://doi.org/10.1039/C3CS60273G
Alafeef M, Dighe K, Moitra P, Pan D (2020) Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano 14:17028–17045. https://doi.org/10.1021/acsnano.0c06392
Rudrapal M, Khairnar SJ, Jadhav A (2020) Drug repurposing: an emerging approach in drug discovery. In: Dekebo A (ed) Drug repurposing—hypothesis, molecular aspects and therapeutic applications. London: IntechOpen, p. 1–20. doi: https://doi.org/10.5772/intechopen.93193
Fang FC, Naccache SN, Greninger AL (2020) The laboratory diagnosis of coronavirus disease 2019—frequently asked questions. Clin Infect Dis 71:2996–3001. https://doi.org/10.1093/cid/ciaa742
World Health O (2019) Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases Interim guidance. WHO. https://doi.org/10.1093/cid/ciaa742
Aouissi HA, Ababsa M, Gaagai A (2021) Review of a controversial treatment method in the fight against COVID-19 with the example of Algeria. Bull Natl Res Cent 45:94. https://doi.org/10.1186/s42269-021-00550-w
Joung J, Ladha A, Saito M, Kim NG, Woolley AE, Segel M, Barretto RP, Ranu A, Macrae RK, Faure G, Ioannidi EI (2020) Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N Engl J Med 383:1492–1494. https://doi.org/10.1056/NEJMc2026172
Brandsma E, Verhagen HJ, van de Laar TJ, Claas EC, Cornelissen M, van den Akker E (2021) Rapid, sensitive, and specific severe acute respiratory syndrome coronavirus 2 detection: a multicenter comparison between standard quantitative reverse-transcriptase polymerase chain reaction and CRISPR-based DETECTR. J Infect Dis 223:206–213. https://doi.org/10.1093/infdis/jiaa641
Rauch JN, Valois E, Ponce-Rojas JC, Aralis Z, Lach RS, Zappa F, Audouard M, Solley SC, Vaidya C, Costello M, Smith H (2021) Comparison of severe acute respiratory syndrome coronavirus 2 screening using reverse transcriptase-quantitative polymerase chain reaction or CRISPR-based assays in asymptomatic college students. JAMA Netw Open 4:e2037129. https://doi.org/10.1001/jamanetworkopen.2020.37129
Behrouzi K, Lin L (2022) Gold nanoparticle based plasmonic sensing for the detection of SARS-CoV-2 nucleocapsid proteins. Biosens Bioelectron 195:113669. https://doi.org/10.1016/j.bios.2021.113669
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ, O’Horo JC, Shoham S (2020) Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19, updated December 23. doi:https://doi.org/10.1093/cid/ciaa1343
Infantino M, Damiani A, Gobbi FL, Grossi V, Lari B, Macchia D, Casprini P, Veneziani F, Villalta D, Bizzaro N, Cappelletti P (2020) Serological assays for SARS-CoV-2 infectious disease: benefits, limitations and perspectives. Isr Med Assoc J 22:203–210
Al Johani S, Hajeer AH (2016) MERS-CoV diagnosis: an update. J Infect Public Health 9:216–219. https://doi.org/10.1016/j.jiph.2016.04.005
Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S (2020) Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 3:CD013705. https://doi.org/10.1002/14651858.CD013705
Prince-Guerra JL, Almendares O, Nolen LD, Gunn JK, Dale AP, Buono SA, Deutsch-Feldman M, Suppiah S, Hao L, Zeng Y, Stevens VA (2021) Evaluation of Abbott BinaxNOW Rapid Antigen Test for SARS-CoV-2 infection at two community-based testing sites - Pima County, Arizona, November 3–17, 2020, MMWR Morb Mortal Wkly Rep 70:100–105. doi:https://doi.org/10.15585/mmwr.mm7003e3external.
Pray IW (2021) Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 Testing at Two University Campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep 69:1642–1647. https://doi.org/10.15585/mmwr.mm695152a3
Cheng MP, Yansouni CP, Basta NE, Desjardins M, Kanjilal S, Paquette K, Caya C, Semret M, Quach C, Libman M, Mazzola L (2020) Serodiagnostics for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med 173:450–460. https://doi.org/10.7326/M20-285
Hanson KE, Caliendo AM, Arias CA, Englund JA, Lee MJ, Loeb M, Patel R, El Alayli A, Kalot MA, Falck-Ytter Y, Lavergne V (2020) Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19. Clin Infect Dis. https://doi.org/10.1093/cid/ciab048
Kelly-Cirino C, Mazzola LT, Chua A, Oxenford CJ, Van Kerkhove MD (2019) An updated roadmap for MERS-CoV research and product development: focus on diagnostics. BMJ Glob Health, 4: e001105. https://doi.org/10.1136/bmjgh-2018-001105
Aldewachi H, Chalati T, Woodroofe MN, Bricklebank N, Sharrack B, Gardiner P (2018) Gold nanoparticle-based colorimetric biosensors. Nanoscale 018(10):18–33. https://doi.org/10.1039/c7nr06367a
Zhu X, Wang X, Han L, Chen T, Wang L, Li H, Li S, He L, Fu X, Chen S, Xing M (2020) Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens Bioelectron 166:112437. https://doi.org/10.1016/j.bios.2020.112437
Rasmi Y, Li X, Khan J, Ozer T, Choi JR (2021) Emerging point-of-care biosensors for rapid diagnosis of COVID-19: current progress, challenges, and future prospects. Anal Bioanal Chem, pp 1–23. https://doi.org/10.1007/s00216-021-03377-6
Ding R, Chen Y, Wang Q, Wu Z, Zhang X, Li B, Lin L (2021) Recent advances in quantum dots-based biosensors for antibiotic detection. J Pharm Anal. https://doi.org/10.1016/j.jpha.2021.08.002
Kalkal A, Pradhan R, Kadian S, Manik G, Packirisamy G (2020) Biofunctionalized graphene quantum dots based fluorescent biosensor toward efficient detection of small cell lung cancer. ACS Appl Bio Mater 3:4922–4932. https://doi.org/10.1021/acsabm.0c00427
Abd Manan FA, Hong WW, Abdullah J, Yusof NA, Ahmad I (2019) Nanocrystalline cellulose decorated quantum dots based tyrosinase biosensor for phenol determination. Mater Sci Eng 99:37–46. https://doi.org/10.1016/j.msec.2019.01.082
Liu J, Ji D, Meng H, Zhang L, Wang J, Huang Z, Chen J, Li J, Li Z (2018) A portable fluorescence biosensor for rapid and sensitive glutathione detection by using quantum dots-based lateral flow test strip. Sens Actuators B Chem 262:486–492. https://doi.org/10.1007/978-1-0716-0463-2_15
Jing X, Zhou D, Sun R, Zhang Y, Li Y, Li X, Li Q, Song H, Liu B (2021) Enhanced Photoluminescence and Photoresponsiveness of Eu3+ Ions Doped CsPbCl3 Perovskite Quantum Dots under High Pressure. Adv Funct Mater 2021:2100930. https://doi.org/10.1002/adfm.202100930
Pardo J, Peng Z, Leblanc RM (2018) Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Molecules 23:378. https://doi.org/10.3390/molecules23020378
Francis AP, Devasena T (2018) Toxicity of carbon nanotubes: A review. Toxicol Ind Health 34:200–210. https://doi.org/10.1177/0748233717747472
Lin YC, Cheng CY, Chen CP, Cheng SH, Chang SY, Hsueh PR (2020) A case of transient existence of SARS-CoV-2 RNA in the respiratory tract with the absence of anti-SARS-CoV-2 antibody response. Int J Infect Dis 96:464–466. https://doi.org/10.1016/j.ijid.2020.05.070
Li X, Wang L, She L, Sun L, Ma Z, Chen M, Hu P, Wang D, Yang F (2018) Immunotoxicity assessment of ordered mesoporous carbon nanoparticles modified with PVP/PEG. Colloids Surf B 171:485–493. https://doi.org/10.1016/j.colsurfb.2018.07.072
Hua Z, Yu T, Liu D, Xianyu Y (2021) Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens Bioelectron, 113076. https://doi.org/10.1016/j.bios.2021.113076
Lv P, Zhou H, Mensah A, Feng Q, Wang D, Hu X, Cai Y, Lucia LA, Li D, Wei Q (2018) A highly flexible self-powered biosensor for glucose detection by epitaxial deposition of gold nanoparticles on conductive bacterial cellulose. Chem Eng J 351:177–188. https://doi.org/10.1016/j.cej.2018.06.098
Su H, Li S, Jin Y, Xian Z, Yang D, Zhou W, Mangaran F, Leung F (2017) Nanomaterial-based biosensors for biological detections. Adv Health Care Technol 3:19–29. https://doi.org/10.2147/AHCT.S94025
Mieszawska AJ, Mulder WJ, Fayad ZA, Cormode DP (2013) Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol Pharm 10:831–847. https://doi.org/10.1021/mp3005885
Banerjee A, Maity S, Mastrangelo CH (2021) Nanostructures for biosensing, with a brief overview on cancer detection, IoT, and the role of machine learning in smart biosensors. Sensors 21:1253. https://doi.org/10.3390/s21041253
Gooding JJ (2006) Biosensor technology for detecting biological warfare agents: Recent progress and future trends. Anal Chim Acta 559:137–151. https://doi.org/10.1016/j.aca.2005.12
Hamdy ME, Del Carlo M, Hussein HA, Salah TA, El-Deeb AH, Emara MM, Pezzoni G, Compagnone D (2018) Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J Nanobiotechnol 16:1–12. https://doi.org/10.1186/s12951-018-0374-x
Hu L, Fu X, Kong G, Yin Y, Meng HM, Ke G, Zhang XB (2020) DNAzyme–gold nanoparticle-based probes for biosensing and bioimaging. J Mater Chem 8:9449–9465. https://doi.org/10.1039/D0TB01750G
Chen T, Sheng A, Hu Y, Mao D, Ning L, Zhang J (2019) Modularization of three-dimensional gold nanoparticles/ferrocene/liposome cluster for electrochemical biosensor. Biosens Bioelectron 124:115–121. https://doi.org/10.1016/j.bios.2018.09.101
Zhao J, Fang S, Liu Y, Zeng L, He Z (2020) A lateral flow biosensor based on gold nanoparticles detects four hemorrhagic fever viruses. Anal Methods 12:5613–5620. https://doi.org/10.1039/D0AY01137A
Negahdari B, Darvishi M, Saeedi AA (2019) Gold nanoparticles and hepatitis B virus. Artif Cells Nanomed Biotechnol 47:455–461. https://doi.org/10.1080/21691401.2018.1553786
Isermann R (2006), Hardware, in CIGR Handbook of Agricultural Engineering Volume VI Information Technology. Edited by CIGR-The International Commission of Agricultural Engineering; Volume Editor, Axel Munack. St. Joseph, Michigan, USA: ASABE. Copyright American Society of Agricultural Engineers. Çevirmenler: Pınar DEMİRCİOĞLU ve İsmail BÖĞREKCİ.
Mohammed AM, Rahim RA, Ibraheem IJ, Loong FK, Hisham H, Hashim U, Al-Douri Y (2014) c. J Nanomater 2014. https://doi.org/10.1155/2014/683460
Khan J, Asoom LI, Khan M, Chakrabartty I, Dandoti S, Rudrapal M, Zothantluanga JH (2021) Evolution of RNA viruses from SARS to SARS-CoV-2 and diagnostic techniques for COVID-19: a review. Beni-Suef Univ J Basic Appl Sci 10:60. https://doi.org/10.1186/s43088-021-00150-7
Hasöksüz M, Kiliç S, Saraç F, Coronaviruses and sars-cov-2. Turk J Med Sci 50:549–556. https://doi.org/10.3906/sag-2004-127
Bhat EA, Sajjad N, Ali A, Aldakeel FM, Mateen A, Alqahtani MS, Syed R (2021) SARS-CoV-2: insight in genome structure, pathogenesis and viral receptor binding analysis—an updated review. Int Immunopharmacol 95:107493. https://doi.org/10.1016/j.intimp.2021.107493
Tombuloglu H, Sabit H, Al-Suhaimi E, Al Jindan R, Alkharsah KR, Development of multiplex real-time RT-PCR assay for the detection of SARS-CoV-2, PLoS One 16: e0250942. https://doi.org/10.1371/journal.pone.0250942
Benda A, Zerajic L, Ankita A, Cleary E, Park Y, Pandey S (2021) cSensors 21:6581
Sharma A, Balda S, Apreja M, Kataria K, Capalash N, Sharma P (2021) COVID-19 diagnosis: current and future techniques. Int J Biol Macromol 193:1835–1844. https://doi.org/10.3390/s21196581
Yan J, Liu Y, Wang Y, Xu X, Lu Y, Pan Y, Guo F, Shi D (2014) Effect of physiochemical property of Fe3O4 particle on magnetic lateral flow immunochromatographic assay. Sens Actuators B Chem 197:129–136. https://doi.org/10.1016/j.snb.2014.02.067
Ernst E, Wolfe P, Stahura C, Edwards KA (2021) Technical considerations to development of serological tests for SARS-CoV-2. Talanta 224:121883. https://doi.org/10.1016/j.talanta.2020.121883
Haarburger D, Pillay TS (2011) Historical perspectives in diagnostic clinical pathology: development of the pregnancy test. J Clin Pathol 64:546–548. https://doi.org/10.1136/jcp.2011.090332
Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F (2018) Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360:439–444. https://doi.org/10.1126/science.aaq0179
Van DH, Russell DA (2016) Optimisation of immuno-gold nanoparticle complexes for antigen detection. J Colloid Interface Sci 471:127–135. https://doi.org/10.1016/j.jcis.2016.03.001
Tang D, Cui Y, Chen G (2013) Nanoparticle-based immunoassays in the biomedical field. Analyst 138:981–990. https://doi.org/10.1039/C2AN36500F
Zhao J, Chen X, Ho KH, Cai C, Li CW, Yang M, Yi C (2021) Nanotechnology for diagnosis and therapy of rheumatoid arthritis: Evolution towards theranostic approaches. Chin Chem Lett 32:66–86. https://doi.org/10.1016/j.cclet.2020.11.048
Lew TT, Aung KM, Ow SY, Amrun SN, Sutarlie L, Ng LF, Su X (2021) Epitope-functionalized gold nanoparticles for rapid and selective detection of SARS-CoV-2 IgG antibodies. ACS Nano 15:12286–12297. https://doi.org/10.1021/acsnano.1c04091
Duan Y, Wang S, Zhang Q, Gao W, Zhang L (2021) Nanoparticle approaches against SARS-CoV-2 infection. Curr Opin Solid State Mater Sci 25:100964. https://doi.org/10.1016/j.cossms.2021.100964
Rosati M, Agarwal M, Hu X, Devasundaram S, Stellas D, Chowdhury B, Bear J, Burns R, Donohue D, Pessaint L, Andersen H (2021) Control of SARS-CoV-2 infection after Spike DNA or Spike DNA+ protein co-immunization in rhesus macaques. PLoS Pathog 17:e1009701. https://doi.org/10.1371/journal.ppat.1009701
Li M, Shan Y, Cai K, Ren W, Sun H, Wu S, Li J, Hong D, Zhang Z, Wang Q, Qin L (2021) Self-assessment of COVID-19 vaccination efficacy using a simple POCT for SARS-CoV-2 S1 protein antibody IgG-IgM. medRxiv. https://doi.org/10.1101/2021.06.27.21258591
Mabrouk MT, Chiem K, Rujas E, Huang WC, Jahagirdar D, Quinn B, Surendran Nair M, Nissly RH, Cavener VS, Boyle NR, Sornberger TA (2021) Lyophilized, thermostable Spike or RBD immunogenic liposomes induce protective immunity against SARS-CoV-2 in mice. Sci Adv 7:eabj1476. https://doi.org/10.1126/sciadv.abj1476
Huang SH, Yang TC, Tsai MH, Tsai IS, Lu HC, Chuang PH, Wan L, Lin YJ, Lai CH, Lin CW (2008) Gold nanoparticle-based RT-PCR and real-time quantitative RT-PCR assays for detection of Japanese encephalitis virus. Nanotechnology 19:405101
Jans H, Huo Q (2012) Gold nanoparticle-enabled biological and chemical detection and analysis. Chem Soc Rev 41:2849–2866. https://doi.org/10.1039/C1CS15280G
Singh J, Sharma S, Nara S (2015) Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chem 170:470–483. https://doi.org/10.1016/j.foodchem.2014.08.092Get
Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Ya Z (2020) Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 92:1518–1524. https://doi.org/10.1002/jmv.25727
Gupta R, Sagar P, Priyadarshi N, Kaul S, Sandhir R, Rishi V, Singhal NK (2020) Nanotechnology-based approaches for the detection of SARS-CoV-2. Front Nanotechnol 2:6. https://doi.org/10.3389/fnano.2020.589832
Paliwal P, Sargolzaei S, Bhardwaj SK, Bhardwaj V, Dixit C, Kaushik A (2020) Grand challenges in bio-nanotechnology to manage the COVID-19 pandemic. Front Nanotechnol 2:5. https://doi.org/10.3389/fnano.2020.571284
Syrowatka A, Kuznetsova M, Alsubai A, Beckman AL, Bain PA, Craig KJ, Hu J, Jackson GP, Rhee K, Bates DW (2021) Leveraging artificial intelligence for pandemic preparedness and response: a scoping review to identify key use cases. NPJ Digital Med 4:1–14. https://doi.org/10.1038/s41746-021-00459-8
Shen Y, Anwar TB, Mulchandani A (2021) Current status, advances, challenges and perspectives on biosensors for COVID-19 diagnosis in resource-limited settings. Sens Actuator A Phys 3:100025. https://doi.org/10.1016/j.snr.2021.100025
Lee D, Lee J (2020) Testing on the move: South Korea’s rapid response to the COVID-19 pandemic. Transp Res Interdiscip Perspect 5:100111. https://doi.org/10.1016/j.trip.2020.100111
Alhalaili B, Popescu IN, Kamoun O, Alzubi F, Alawadhia S, Vidu R (2020) Nanobiosensors for the detection of novel coronavirus 2019-nCoV and other pandemic/epidemic respiratory viruses: a review. Sensors 20:6591. https://doi.org/10.3390/s20226591
Rai M, Bonde S, Yadav A, Bhowmik A, Rathod S, Ingle P, Gade A (2021) Nanotechnology as a shield against COVID-19: current advancement and limitations. Viruses 13:1224. https://doi.org/10.3390/v13071224
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JK, YR, KKK, AA, MR, and RRP contributed to writing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, J., Rasmi, Y., Kırboğa, K.K. et al. Development of gold nanoparticle-based biosensors for COVID-19 diagnosis. Beni-Suef Univ J Basic Appl Sci 11, 111 (2022). https://doi.org/10.1186/s43088-022-00293-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00293-1