Abstract
Background
Cerebral blood flow (CBF) plays an important role in neurological recovery after cardiac arrest (CA) resuscitation. However, the variations of CBF recovery in distinct brain regions and its correlation with neurologic recovery after return of spontaneous circulation (ROSC) have not been characterized. This study aimed to investigate the characteristics of regional cerebral reperfusion following resuscitation in predicting neurological recovery.
Methods
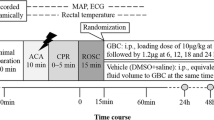
Twelve adult male Wistar rats were studied, ten resuscitated from 7-min asphyxial CA and two uninjured rats, which were designated as healthy controls (HCs). Dynamic changes in CBF in the cerebral cortex, hippocampus, thalamus, brainstem, and cerebellum were assessed by pseudocontinuous arterial spin labeling magnetic resonance imaging, starting at 60 min after ROSC to 156 min (or time to spontaneous arousal). Neurologic outcomes were evaluated by the neurologic deficit scale at 24 h post-ROSC in a blinded manner. Correlations between regional CBF (rCBF) and neurological recovery were undertaken.
Results
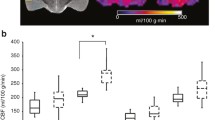
All post-CA animals were found to be nonresponsive during the 60–156 min post ROSC, with reductions in rCBF by 24–42% compared with HC. Analyses of rCBF during the post-ROSC time window from 60 to 156 min showed the rCBF recovery of hippocampus and thalamus were positively associated with better neurological outcomes (rs = 0.82, p = 0.004 and rs = 0.73, p < 0.001, respectively). During 96 min before arousal, thalamic and cortical rCBF exhibited positive correlations with neurological recovery (rs = 0.80, p < 0.001 and rs = 0.65, p < 0.001, respectively); for predicting a favorable neurological outcome, the thalamic rCBF threshold was above 50.84 ml/100 g/min (34% of HC) (area under the curve of 0.96), whereas the cortical rCBF threshold was above 60.43 ml/100 g/min (38% of HC) (area under the curve of 0.88).
Conclusions
Early magnetic resonance imaging analyses showed early rCBF recovery in thalamus, hippocampus, and cortex post ROSC was positively correlated with neurological outcomes at 24 h. Our findings suggest new translational insights into the regional reperfusion and the time window that may be critical in neurological recovery and warrant further validation.





Similar content being viewed by others
References
Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-528.
Mallikethi-Reddy S, Briasoulis A, Akintoye E, et al. Incidence and survival after in-hospital cardiopulmonary resuscitation in nonelderly adults: US experience, 2007 to 2012. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003194.
Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79(3):350–79.
Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21(1):90.
Kawai K, Nitecka L, Ruetzler CA, et al. Global cerebral ischemia associated with cardiac arrest in the rat: I. Dynamics of early neuronal changes. J Cereb Blood Flow Metab. 1992;12(2):238–49.
Hendrickx HHL, Rao GR, Safar P, Gisvold SE. Asphyxia, cardiac arrest and resuscitation in rats. I. Short term recovery. Resuscitation. 1984;12(2):97–116.
Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15(6):1032–9.
Wang Q, Miao P, Modi HR, Garikapati S, Koehler RC, Thakor NV. Therapeutic hypothermia promotes cerebral blood flow recovery and brain homeostasis after resuscitation from cardiac arrest in a rat model. J Cereb Blood Flow Metab. 2019;39(10):1961–73.
Iordanova B, Li L, Clark RSB, Manole MD. Alterations in cerebral blood flow after resuscitation from cardiac arrest. Front Pediatr. 2017;5:174.
Drabek T, Foley LM, Janata A, et al. Global and regional differences in cerebral blood flow after asphyxial versus ventricular fibrillation cardiac arrest in rats using ASL-MRI. Resuscitation. 2014;85(7):964–71.
Patil KD, Halperin HR, Becker LB. Cardiac arrest: resuscitation and reperfusion. Circ Res. 2015;116(12):2041–9.
Wei Z, Wang Q, Modi HR, et al. Acute-stage MRI cerebral oxygen consumption biomarkers predict 24-hour neurological outcome in a rat cardiac arrest model. NMR Biomed. 2020;33(11):e4377.
Geocadin RG, Muthuswamy J, Sherman DL, Thakor NV, Hanley DF. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov Disord. 2000;15(S1):14–21.
Modi HR, Wang Q, Gd S, et al. Intranasal post-cardiac arrest treatment with orexin-A facilitates arousal from coma and ameliorates neuroinflammation. PLoS ONE. 2017;12(9):e0182707.
Geocadin RG, Ghodadra R, Kimura T, et al. A novel quantitative EEG injury measure of global cerebral ischemia. Clin Neurophysiol. 2000;111(10):1779–87.
Muthuswamy J, Kimura T, Ding MC, Geocadin R, Hanley DF, Thakor NV. Vulnerability of the thalamic somatosensory pathway after prolonged global hypoxic–ischemic injury. Neuroscience. 2002;115(3):917–29.
Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102–16.
Hirschler L, Debacker CS, Voiron J, Köhler S, Warnking JM, Barbier EL. Interpulse phase corrections for unbalanced pseudo-continuous arterial spin labeling at high magnetic field. Magn Reson Med. 2018;79(3):1314–24.
Hirschler L, Munting LP, Khmelinskii A, et al. Transit time mapping in the mouse brain using time-encoded pCASL. NMR Biomed. 2018;31(2):e3855.
Aslan S, Xu F, Wang PL, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 2010;63(3):765–71.
Geocadin RG, Sherman DL, Christian Hansen H, et al. Neurological recovery by EEG bursting after resuscitation from cardiac arrest in rats. Resuscitation. 2002;55(2):193–200.
Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676–86.
Becker LB, Aufderheide TP, Geocadin RG, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124(19):2158–77.
Manole MD, Kochanek PM, Bayır H, et al. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental cardiac arrest in rats. Pediatr Res. 2014;75(2):295–301.
Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. 2010;26(1):5–13.
Li C-X, Patel S, Wang DJJ, Zhang X. Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn Reson Imaging. 2014;32(7):956–60.
Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. Management of brain injury after resuscitation from cardiac arrest. Neurol Clin. 2008;26(2):487–506.
Björklund E, Lindberg E, Rundgren M, Cronberg T, Friberg H, Englund E. Ischaemic brain damage after cardiac arrest and induced hypothermia—a systematic description of selective eosinophilic neuronal death. A neuropathologic study of 23 patients. Resuscitation. 2014;85(4):527–32.
Brisson CD, Hsieh Y-T, Kim D, Jin AY, Andrew RD. Brainstem neurons survive the identical ischemic stress that kills higher neurons: insight to the persistent vegetative state. PLOS ONE. 2014;9(5):e96585.
Ito U, Spatz M, Walker JT, Klatzo I. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol. 1975;32(3):209–23.
Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40(3):599–636.
Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239(1):57–69.
Sadowski M, Wisniewski HM, Jakubowska-Sadowska K, Tarnawski M, Lazarewicz JW, Mossakowski MJ. Pattern of neuronal loss in the rat hippocampus following experimental cardiac arrest-induced ischemia. J Neurol Sci. 1999;168(1):13–20.
Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37(5):1334–9.
Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6.
Foley LM, Clark RSB, Vazquez AL, et al. Enduring disturbances in regional cerebral blood flow and brain oxygenation at 24 hours after asphyxial cardiac arrest in developing rats. Pediatr Res. 2017;81(1–1):94–8.
Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–18.
Forgacs PB, Frey H-P, Velazquez A, et al. Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Ann Clin Transl Neurol. 2017;4(2):119–29.
Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–85.
Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71(6):531–46.
Jang SH, Hyun YJ, Lee HD. Recovery of consciousness and an injured ascending reticular activating system in a patient who survived cardiac arrest. Medicine (Baltimore). 2016;95(26):e4041.
Snider SB, Bodien YG, Frau-Pascual A, Bianciardi M, Foulkes AS, Edlow BL. Ascending arousal network connectivity during recovery from traumatic coma. Neuroimage Clin. 2020;28:102503.
Snider SB, Bodien YG, Bianciardi M, Brown EN, Wu O, Edlow BL. Disruption of the ascending arousal network in acute traumatic disorders of consciousness. Neurology. 2019;93(13):e1281–7.
Brunko E, de Beyl DZ. Prognostic value of early cortical somatosensory evoked potentials after resuscitation from cardiac arrest. Electroencephalogr Clin Neurophysiol. 1987;66(1):15–24.
Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61(3):461–70.
Pollock JM, Whitlow CT, Deibler AR, et al. Anoxic injury-associated cerebral hyperperfusion identified with arterial spin-labeled MR imaging. AJNR Am J Neuroradiol. 2008;29(7):1302–7.
Manchester LC, Lee V, Schmithorst V, Kochanek PM, Panigrahy A, Fink EL. Global and regional derangements of cerebral blood flow and diffusion magnetic resonance imaging after pediatric cardiac arrest. J Pediatr. 2016;169:28-35.e1.
Jia X, Koenig MA, Shin H-C, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76(3):431–42.
Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–50.
Hossmann K-A. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36(4):557–65.
Funding
This work was supported by National Institutes of Health grants R01 HL071568-15, R01 HL139158-01A1, R01 AG064792, and R21 AG058413.
Author information
Authors and Affiliations
Contributions
RGG: supervised the project, provided research idea, reviewed the article, and served as the co-principal investigator. NVT: supervised the project, reviewed the article, and served as the co-principal investigator. YG: conducted data analyses and prepared the article. SMC: provided research idea and reviewed the article. ZW, QW, and HM: conducted animal experiments and data collection. PG: consulted and reviewed/edited the article. HL: supervised the magnetic resonance imaging study. All authors contributed to article revisions and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declared that they have no conflicts of interest.
Ethical approval/human and animal rights
This study was in compliance with ethical standards for animal studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, Y., Cho, SM., Wei, Z. et al. Early Thalamocortical Reperfusion Leads to Neurologic Recovery in a Rodent Cardiac Arrest Model. Neurocrit Care 37, 60–72 (2022). https://doi.org/10.1007/s12028-021-01432-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01432-9