Abstract
A thyroid nodule classified as indeterminate on fine-needle aspiration cytology (FNAC), hereafter referred to as an indeterminate thyroid nodule (ITN), represents a clinical dilemma. The Italian Consensus for the Classification and Reporting of Thyroid Cytology (ICCRTC) divides ITNs into low- and high-risk categories (i.e., TIR3A and TIR3B, respectively) to better manage patients. This study aimed to achieve high-evidence estimates of the prevalence, rate of operation, and risk of malignancy of ITNs, including TIR3A and TIR3B ITNs. This systematic review was conducted according to MOOSE to retrieve all original studies citing ICCRTC. The last search was performed in February 2022. The risk of bias of the included studies was assessed. Separate proportion meta-analyses were performed with a random-effect model using OpenMeta[Analyst]. The online search processed 271 studies, and 33 were finally considered. First, the cancer prevalence among ITNs was 32.4%. Second, the cancer prevalence among TIR3As was 12.4%, with heterogeneity (I2 90%) explained by a linear correlation between sample size and cancer rate (p = 0.009). Third, the cancer prevalence among TIR3Bs was 44.4%, with heterogeneity (I2 75%) explained by the inverse correlation between sample size and cancer rate (p = 0.031). Fourth, the prevalence of ITNs, TIR3A, and TIR3B among FNACs was 29.6%, 12.6%, and 12.9%, respectively, with sample size and TIR3B prevalence being inversely correlated (p = 0.04). Fifth, the operation rates of ITNs, TIR3A, and TIR3B were 54.3%, 48.3%, and 75.2%, respectively, and the sample size and TIR3A operation rate were inversely correlated (p = 0.010). These data strongly support the division of ITNs into low- and high-risk subcategories. Importantly for clinical practice, the cancer rate among ITNs is significantly influenced by the study sample size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid nodules classified as indeterminate on fine-needle aspiration cytology (FNAC), hereafter referred to as indeterminate thyroid nodules (ITNs), represent one of the most relevant clinical dilemmas in the field of clinical thyroidology. Thyroid nodule is a largely diffuse pathological entity that is often incidentally discovered during neck imaging performed following nonthyroidal indications [1]. According to international guidelines [2, 3], in patients with newly discovered thyroid nodule(s), the indication for further diagnostic procedures should be considered. In this context, ultrasound (US)-guided fine-needle aspiration cytology (FNAC) is recognized as the most reliable tool [2, 3]. In fact, FNAC is able to discriminate malignant from benign thyroid nodules with high accuracy. However, a nonnegligible number of FNACs are classified as ITNs, namely a kind of nodule in which a full diagnosis can be achieved only by histological evaluation after surgery. Since the prevalence of ITNs among FNACs is expected to be 20 to 25% and considering that approximately one in three ITNs is expected to be cancer [4], international guidelines recommend managing these patients according to specific clinical context and US features with the aim of avoiding surgeries as much as possible. Then, the indeterminate category is usually divided into two subcategories, such as Thy 3a (ITN with atypia) and Thy 3f (ITN with follicular pattern) in the UK Royal College of Pathologists (RCPath) guidelines [5], AUS/FLUS (atypia of undetermined significance/follicular lesion of undetermined significance), and FN/SFN (follicular neoplasm/suspicious for a follicular neoplasm) in The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) [6], and TIR3A and TIR3B in Italian consensus for the classification and reporting of thyroid cytology (ICCRTC) [7]. Some systematic reviews with meta-analyses have been published about the rate of malignancy of these subcategories, and they found a cancer rate of 25% (95% CI 20 to 31) for Thy 3a and 31% (95% CI 24 to 39) Thy 3f [8] in UK RCPath, 30.5% (95% CI 24.2–37.0) [9] or 27% (95% CI 23 to 31) [10] in AUS/FLUS, 28.9% (95% CI 26.2–31.6) [9] or 31% (95% CI 28 to 36) [10] in FN/SFN of TBSRTC, 17% (95% CI 12 to 22) in TIR3A, and 47% (95% CI 40 to 55) in TIR3B [11] of ICCRTC. While from a clinical standpoint these results seem to help to accurately guide the management of ITN patients, we must consider that they were obtained only from a series of patients managed and operated in each single institution according to institution-specific clinical guidelines, international or national guidelines, and other factors, such as endocrinologists’ and surgeons’ expertise and patient preference. Then, we must ask ourselves how this selection bias could influence the findings forming the international guidelines. In addition, we have to take into account that ITNs can include highly aggressive follicular carcinoma, which is difficult to identify on US [12] and is not detectable in cytological samples [4].
Following the above critical issues, the present systematic review was undertaken to achieve more robust information about the risk of malignancy among ITNs. Theoretically, to assess the true cancer prevalence among ITNs, we should operate on all cases. Since this is not possible in clinical practice, we could better understand the cancer risk of ITNs considering several variables as influencing factors on the prevalence of malignancy recorded among the subgroup of operated patients, including the study design (with or without the revision and reclassification of FNAC samples), the overall number of consecutive nodules with available FNAC in a specific period, the prevalence of ITN subcategories among all FNACs, the operation rate, and the final diagnosis at the time of histological assessment. Considering this background, we aimed to properly estimate the prevalence, rate of operation, and risk of malignancy of the indeterminate category of ICCRTC, with the latter being the most reliable system for discriminating low- from high-risk ITNs [11].
Materials and Methods
Conduct of Review
This review was conducted according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [13].
Search Strategy
A specific strategy to retrieve all original studies citing ICCRTC was planned. Accordingly, the online citation databases Google Scholar and Scopus were searched to find the largest possible number of papers citing ICCRTC. No language restriction was used. A beginning date limit was not used. The last search was performed on February 26, 2022. Additionally, the reference lists of the studies were screened to select additional articles.
Study Selection
Records found according to the above strategy were fully screened. Original papers reporting data of ITN according to ICCRTC 2014 were included. Articles were not included if (a) they did not cover the field of interest of this systematic review; (b) the histological findings of ITNs was not available; or (c) the data overlapped with other studies. In addition, review articles, editorials, letters, case/series reports (< 10 cases) and pediatric studies were always excluded. Two authors (GF, PT) autonomously reviewed the abstracts of the articles and selected those eligible. In case of disagreement, a consensus was achieved after collegial discussion with the other authors.
Data Extraction
The following information was extracted independently by two authors (GF, MC) from each study: (1) general study information (authors’ name, year of publication and country origin); (2) modality of enrollment of data of FNACs according to ICCRTC (prospective using ICCRTC during clinical practice or retrospective reclassifying according to ICCRTC of all FNACs performed before 2014); (3) overall number of FNACs performed during the study period; (4) number of ITNs found during the study period; (5) number of ITNs operated on during the study period; (6) number of cancers and benign lesions among ITNs operated on. Separate data extractions were performed for overall ITN, TIR3A and TIR3B. Missing data were obtained from authors of original papers, when appropriate. Data were cross-checked, and a collegial discussion among the authors resolved any discrepancies when present.
Study Quality Assessment
The risk of bias was independently evaluated by two authors (MC, PT) for each study according to the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Studies [14].
Statistical Analysis
The primary outcomes were (1) the prevalence of cancer among ITNs, TIR3A, and TIR3B; (2) the operation rate among ITNs, TIR3A, and TIR3B; and (3) the prevalence of ITNs, TIR3A and TIR3B among all FNACs. Separate proportion meta-analyses were performed using the DerSimonian and Laird method (random-effect model) [15], where pooled data represent weighted averages according to study sample size. Forest plots displayed the pooled data with 95% confidence intervals (95% CI). The I2 index was used to evaluate inconsistencies, assessing them as follows: < 25% indicated no heterogeneity, 25–50% indicated mild heterogeneity, 50–75% indicated moderate heterogeneity, and > 75% indicated high heterogeneity. To explore heterogeneity, subgroup analyses and meta-regression analyses were attempted using appropriate covariates (i.e., modality of enrollment of FNAC data according to ICCRTC and sample size). A p < 0.05 was regarded as significant. Statistical analyses were performed using OpenMeta[Analyst] (open-source software developed by the Center for Evidence Synthesis in Health, Brown University, Providence, RI, USA).
Results
Eligible Articles
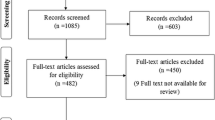
After excluding duplicates, the online search retrieved 271 articles. According to the above selection criteria, 62 articles were initially selected, and 33 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] were finally included in the systematic review (Fig. 1).
Qualitative Analysis (Systematic Review)
The 33 articles were published between 2014 and 2021 in scientific journals in the fields of endocrinology (n = 23), cytopathology (n = 3), medicine (n = 3), oncology (n = 2), surgery (n = 1), and radiology (n = 1). The seven oldest studies included nodules originally classified as TIR3 [49], and all cases were reclassified as TIR3A or TIR3B according to the 2014 ICCRTC [7]. Nineteen studies considered nodules first classified as TIR3A or TIR3B, and the remaining 7 papers reported both cases. The overall number of FNACs performed during the study period was available in 20 studies. The total number of ITNs operated on with histological follow-up was 4940, and there were 1516 cases of cancer. Tables 1 and 2 illustrate the main characteristics and full data of the 33 studies.
Study Quality Assessment
The assessment of the risk of bias of each study is detailed in the Supplemental data. For all articles, statement of the study question, inclusion and exclusion criteria, exposure of interest (i.e., FNAC), timeframe between exposure and outcome (i.e., histology), and outcome measures were adequate. In two studies, the population was not properly defined [16, 28]. Sample size justification was never reported. Whether the participation rate of eligible persons was at least 50%, it was unclear in 16 studies [16, 17, 19, 24, 26, 28, 30, 33, 35, 37, 41,42,43,44,45,46]. A loss to follow-up after baseline below 20% was reported in 16 studies [16,17,18,19,20, 22, 23, 27, 30, 31, 34,35,36, 40, 43, 45].
Quantitative Analysis (Meta-analysis)
First, the pooled prevalence of cancer among all ITNs was evaluated, and a rate of 32.4% (95% CI 29.2–35.5) was found with high heterogeneity (I2 78%). Neither study design (i.e., studies with nodules classified as TIR3A or TIR3B during clinical practice vs. the other ones) nor sample size could explain this finding. However, when the largest study [48] was excluded, an inverse correlation was found between sample size and cancer rate (p = 0.025): the higher the sample size was, the lower the cancer rate.
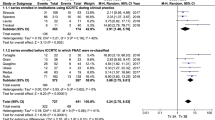
Second, the pooled group of 2626 TIR3A cases was analyzed. The cancer prevalence was 12.4% (95% CI 8.8–15.9), with high heterogeneity (I2 90%). As described above, heterogeneity was explored according to the study design and sample size. Concerning the former aspect, there was no difference between the subgroup of studies reporting data of nodules reclassified as TIR3A and that of studies including nodules assessed as TIR3A during clinical practice. Regarding the sample size, the meta-regression analysis found a significant linear correlation between sample size and cancer rate (p = 0.009): the higher the sample size was, the higher the cancer rate (Fig. 2). However, this result depended on the high weight of the largest study [48], without a significant difference after excluding that series.
Third, the pooled group of 2314 TIR3B nodules was investigated. The cancer prevalence in this category was 44.4% (95% CI 40.1–48.8) with moderate heterogeneity (I2 75%). The heterogeneity was explored as described above according to study design and sample size. The study design did not explain the heterogeneity. However, the meta-regression considering the sample size showed a significant inverse correlation between sample size and cancer rate (p = 0.031): the higher the sample size was, the lower the cancer rate (Fig. 3). Since the largest study [48] influenced the results of ITN and TIR3A, this was also verified in TIR3B; when excluding that study, the significance of the correlation increased (p = 0.001).
Fourth, the prevalence of ITN, TIR3A, and TIR3B among all FNACs was analyzed. Among those 20 studies reporting the overall number of biopsies performed during the study period, after excluding papers reporting only FNACs with ITN results, there were 16 studies eligible for this analysis [21, 25, 27,28,29, 32,33,34,35, 37, 40, 42,43,44, 47]. Overall, the prevalence of ITNs among FNACs was 29.6% (95% CI 25–34.1), with high heterogeneity (I2 98%). When sample size was used as a covariate, a significant inverse correlation was found between the study sample and ITN prevalence (p = 0.002): the higher the sample size was, the lower the ITN prevalence. The pooled prevalence of TIR3A among FNACs was 12.6% (95% CI 10.1–15.2), with high heterogeneity (I2 96%), remaining unexplained by meta-regression analysis using sample size as a covariate (p = 0.14). The pooled prevalence of TIR3B among FNACs was 12.9% (95% CI 10.5–15.3), with high heterogeneity (I2 97%). When sample size was used as a covariate, a significant inverse correlation was observed between sample size and TIR3B prevalence (p = 0.04): the higher the sample size was, the lower the prevalence of TIR3B (Fig. 4).
Fifth, the operation rates of ITN, TIR3A, and TIR3B were analyzed. For this analysis, 12 studies were eligible [21, 25, 27, 29, 34, 35, 39,40,41,42, 46, 47]. The operation rate of all ITNs was 54.3% (95% CI 38.2–70.5) with high heterogeneity (I2 99%), leaving the latter unexplained when performing a meta-regression analysis using sample size as a covariate (p = 0.20). When considering the TIR3A group, the operation rate was 48.3% (95% CI 21.9–74.6), with high heterogeneity (I2 99%). The latter was explored using the sample size of TIR3A, and a significant inverse correlation was observed between sample size and TIR3A operation rate (p = 0.010): the higher the sample size was, the lower the operation rate (Fig. 5). When analyzing the TIR3B group, the operation rate was 75.2% (95% CI 65.9–84.5), with high heterogeneity (I2 98%), leaving the latter unexplained when performing a meta-regression analysis using sample size as a covariate.
Finally, the main findings of the present study are summarized in Table 3.
Discussion
ITN is still a challenge in cytopathology since morphology alone is not able to classify these lesions. Additionally, even if ancillary molecular testing (from single mutational assessment to broader genetic panels) might contribute to more precise and tailored patient management, their use is limited due to their costs. Currently, addressing ITN is still clinically problematic. We can tell our patient that the risk of malignancy is not high, probably mild-to-moderate, even if a cancer cannot be excluded until he is operated upon. The present systematic review aimed to investigate the size of the ITNs. In particular, this study evaluated the prevalence of ITNs among thyroid nodules selected for FNAC, how many patients with ITNs are operated upon, and how many ITNs are malignant once patients are operated upon. Implicitly, these analyses might allow us to better understand the true risk of malignancy of these cases.
First, it should be emphasized that the present systematic review retrieved 271 articles citing ICCRTC, while a previous review [11] found only 95 records. This means that the interest of researchers in ICCRTC is rapidly increasing over time. In addition, while the previous meta-analysis [11] included 1168 ITNs with histological follow-up from 10 studies, we included 4940 cases from 33 studies. This large number of cases should enable us to better illustrate the dimension of ITNs and analyze several aspects. Remarkably, the present systematic review found full data about the flow of ITNs in clinical practice, i.e., their prevalence among FNACs, the resection rate among these patients, and the cancer prevalence among those operated on, and this allowed us to estimate how the cancer rate of ITNs found in histological examination (at the end of the flow) changes according to various covariates. This kind of data could increase the generalizability of the results. In fact, in the field of meta-analyses, the largest the number of covariates available to explore in the pooled results, the more significant the findings. Indeed, the present data form a solid reference for the revised version of ICCRTC. Table 4 compares main data and results of the two studies. It is important to underline that, as a consequence of the larger number of cases, the CIs of the present study were shorter than that of the previous study.
First, while 32.4% of all ITNs were found to be cancerous once patients underwent surgery, a significant difference was found between TIR3A and TIR3B, where the cancer rates were 12.4 and 44.4%, respectively. This finding is of high interest in the current era, in which international terminology harmonization and standardization should be required [50]. In fact, the meta-analyses focused on other FNAC reporting systems did not find a different risk of malignancy between the subclasses of ITN [8,9,10]. Table 5 summarizes the pooled results obtained in the major meta-analyses about the three major systems of thyroid FNAC. From this point of view, the most relevant difference between ICCRTC and both TBSRTC [6] and UK RCPath [5] is the classification of nuclear atypia. The latter are put into AUS/FLUS of TBSRTC [6] and Thy 3a of UK RCPath [5], which did not aim to separate the subclasses of ITNs according to their risk of malignancy. In contrast, ICCRTC categorized nuclear atypia with potential to be associated with papillary thyroid carcinoma into the “high-risk” category of TIR3B and the other atypia into the “low-risk” TIR3A [7]. Figure 6 illustrates the cytological presentation of two cases of TIR3A and TIR3B with their final histological diagnosis. In this context, it is worth noting a meta-analysis evaluating aspirates with nuclear/cytologic atypia [51] and reporting their significantly higher risk of malignancy. In addition, it has to be mentioned that the risk of malignancy among the subcategories of AUS/FLUS varies significantly, ranging from 15% in “Hürthle cell aspirates with low-risk pattern” to 44% in “Focal cytologic atypia” [52]. Since mild nuclear atypia has been considered in the Bethesda IV class (FN/SFN) of the last TBSRTC version [53], further studies are needed to analyze its impact in clinical practice.
Two cases of thyroid nodules cytologically classified as indeterminate. The upper figures illustrate a nodule classified as TIR3A. Left: Several microfollicular clusters may be observed in a blood-stained background. The cell groups show a certain degree of monotony with slightly enlarged nuclei and finely irregular chromatin. No clear-cut nuclear grooves or intranuclear cytoplasmic inclusions (INCI) are noticed. Right: the postsurgical histological sample showed a follicular variant papillary carcinoma with follicular-patterned lesion where thyrocytes show enlarged nuclei with chromatin clearing and occasional nuclear grooves and INCI. Nuclei also show a tendency to overlap. The follicular lumens contain dense colloid. The lower figures illustrate a TIR3B case. Left: the cytological picture shows abundant cellularity organized into microfollicular structures or trabeculae. Thyrocytes show nucleocytoplasmic atypia with enlarged and pleomorphic nuclei with granular chromatin. The cytoplasm is moderately or well represented, sometimes showing a granular appearance. Colloid is scant. Right: the postsurgical histology showed a classical papillary thyroid carcinoma, made up of papillary clusters of thyrocytes with enlarged nuclei, overlapping and chromatic clearing. Moreover, INCI, nuclear grooves and small nucleoli can be seen
Second, the most important novel information found in the present systematic review is that there is a strong impact of study sample size on the cancer rate among ITNs, their prevalence among all FNACs and, remarkably, the rate of ITN patients operated upon. Specifically, the prevalence of ITNs, TIR3A and TIR3B among all FNAC was 29.6%, 12.6%, and 12.9%, respectively, while the prevalence of operated nodules was 54.3%, 48.3%, and 75.2%, respectively. Additionally, when we evaluated the impact of sample size on these findings, we observed that the higher the size was, (a) the lower the prevalence of ITNs and TIR3B among FNACs; (b) the lower the operation rate of patients with TIR3A; (c) the lower the cancer rate in TIR3B cases; and (d) the higher the cancer rate in TIR3A. Several variables, such as (a) the different management of any single patient with ITN (and thyroid nodule, of course) in large- and small-volume institutions, (b) the expertise of institutional endocrinologists, pathologists and surgeons, (c) the availability of second-line diagnostic techniques to be used in ITNs (i.e., molecular markers, core biopsy, PET/CT, and other), (d) the rate of patients lost at follow-up, and (e) the preference of patients, could have influenced these findings.
Third, because of these issues, ICCRTC recommendations should be addressed. The suggested actions by ICCRTC are (1) to plan an active clinical observation as the first option in most TIR3A cases with repeated FNAC over time and (2) to operate on patients with TIR3B as the main option. In addition, in the ICCRTC document, since no published data exist regarding both the frequency of ITNs and the risk of malignancy, attempts should be made to keep the TIR3A and TIR3B frequencies < 10%, each with an expected cancer rate < 10% in TIR3A and between 10 and 20% in TIR3B. Based on the data recorded herein, we can affirm that these suggested actions are not fully followed in clinical practice, especially in small-size studies. In fact, more than half of ITNs are addressed via surgery, with a resection rate of 48.3% among TIR3A cases. In addition, the ITN prevalence among FNACs was approximately one-third, with a significant interaction between the TIR3B prevalence and the study sample size.
A comprehensive discussion of these findings is needed. Theoretically, we can expect that a small-size study reports highly selected case series with a potential bias in terms of overestimation of cancer: the smaller the series of ITNs, the more accurate the clinical selection of cases at high risk of cancer (e.g., suspicious US), the higher the operation rate, and the higher the cancer rate at the time of histological examination. From the researchers’ point of view, we have to take into account that, generally, small-sample studies report a positive correlation, which encourages authors (and journal editors) to publish those data. From the clinicians’ standpoint, the creators of guidelines should carefully consider data derived from large-sample studies. As mentioned above, in the 2014 version of ICCRTC, the obvious absence of clinical data on the frequency and cancer rate of TIR3A and TIR3B was underlined. The present findings allow us to obtain solid information about both references. In fact, the frequency of TIR3A and TIR3B was found to be just above 10% among all FNACs, as initially estimated in ICCRTC. However, the cancer rates of TIR3A and TIR3B were quite different from those expected by the ICCRTC board. The results recorded herein can constitute a basis on which to better estimate the frequency of ITNs among FNACs and the risk of malignancy of the two subclasses.
As is typical in systematic reviews, both limitations and strengths of data should be discussed. First, a large number of papers included a retrospective series of ITNs that were reclassified as TIR3A or TIR3B for the study aim. However, data from these studies did not significantly vary from those obtained when pooling studies including nodules classified as TIR3A and TIR3B in clinical practice. Second, those studies with small sample size could have a significant selection bias (in patients with ITNs, in those operated upon, and in those with an initial diagnosis of thyroid nodules). However, this was fully explored and clearly explained in the present study. Third, almost all studies retrieved in the present systematic review were, as largely expected, from Italian authors. Although these results cannot be extended to other countries, they are reliable, as they were derived from institutes that use ICCRTC in their routine clinical practice. Forth, data about non-invasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP) did not allow any exploration according to operation rate and other covariates. This was due to the fact that NIFTP was not included in the ICCRTC [54]. Then, while the distribution of NIFTP over TBSRTC categories is known [55], its impact on ICCRTC remains unclear.
In conclusion, the present meta-analysis included a very large number of ITNs and corroborates that the cancer rate among ITNs is 32.4%, with a significant difference between low- and high-risk subcategories. Furthermore, this study found that the overall prevalence of ITNs among FNACs was 29.6%, the resection rate of patients with ITNs was 54.3%, and the cancer rate among ITNs was significantly influenced by the study sample size. We advise that the revised version of ICCRTC takes into account these findings as a reference.
References
Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS (2018) The diagnosis and management of thyroid nodules: a review. JAMA 319:914–924. https://doi.org/10.1001/jama.2018.0898
Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, Vitti P; AACE/AME/ETA Task Force on Thyroid Nodules (2010) American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules: executive summary of recommendations. Endocr Pract 33:1-50. https://doi.org/10.4158/EP.16.3.468
Haugen BR, Alexander EK, Bible KC et al (2015) American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1- 133. https://doi.org/10.1089/thy.2015.0020
Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK (2002) Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol 26:41-44. https://doi.org/10.1002/dc.10043.
Perros P, Boelaert K, Colley S et al (2014) Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 81:1-122. https://doi.org/10.1111/cen.12515
Cibas ES, Ali SZ (2009) The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19:1159-1165. https://doi.org/10.1089/thy.2009.0274
Nardi F, Basolo F, Crescenzi A et al (2014) Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest 37:593-599. https://doi.org/10.1007/s40618-014-0062-0
Poller DN, Bongiovanni M, Trimboli P (2020) Risk of malignancy in the various categories of the UK Royal College of Pathologists Thy terminology for thyroid FNA cytology: a systematic review and meta-analysis. Cancer Cytopathol 128:36-42. https://doi.org/10.1002/cncy.22201
Vuong HG, Ngo HTT, Bychkov A, Jung CK, Vu TH, Lu KB, Kakudo K, Kondo T (2020) Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: A systematic review and meta-analysis. Cancer Cytopathol 128:238-249. https://doi.org/10.1002/cncy.22228
Straccia P, Rossi ED, Bizzarro T, Brunelli C, Cianfrini F, Damiani D, Fadda G (2015) A meta-analytic review of the Bethesda System for Reporting Thyroid Cytopathology: has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol 123:713–722. https://doi.org/10.1002/cncy.21605
Trimboli P, Crescenzi A, Giovanella L (2018) Performance of Italian Consensus for the Classification and Reporting of Thyroid Cytology (ICCRTC) in discriminating indeterminate lesions at low and high risk of malignancy. A systematic review and metaanalysis. Endocrine 60:31–35. https://doi.org/10.1007/s12020-017-1382-6
Castellana M, Piccardo A, Virili C, Scappaticcio L, Grani G, Durante C, Giovanella L, Trimboli P (2020) Can ultrasound systems for risk stratification of thyroid nodules identify follicular carcinoma? Cancer Cytopathol. 128:250-259. https://doi.org/10.1002/cncy.22235
Stroup DF, Berlin JA, Morton SC et al (2020) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283:2008-2012. https://doi.org/10.1001/jama.283.15.2008
National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 20 March 2022.
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials. 7:177-188. https://doi.org/10.1016/0197-2456(86)90046-2
Agretti P, Niccolai F, Rago T et al (2014) BRAF mutation analysis in thyroid nodules with indeterminate cytology: our experience on surgical management of patients with thyroid nodules from an area of borderline iodine deficiency. J Endocrinol Invest. 37:1009-1014. https://doi.org/10.1007/s40618-014-0166-6
Rossi ED, Bizzarro T, Martini M, Capodimonti S, Sarti D, Cenci T, Bilotta M, Fadda G, Larocca LM (2016) The evaluation of miRNAs on thyroid FNAC: the promising role of miR-375 in follicular neoplasms Endocrine 54:723–732. https://doi.org/10.1007/s12020-016-0866-0
Grani G, Lamartina L, Ascoli V et al (2017) Ultrasonography scoring systems can rule out malignancy in cytologically indeterminate thyroid nodules. Endocrine 57:256-261. https://doi.org/10.1007/s12020-016-1148-6
Tartaglia F, Giuliani A, Tromba L et al (2016) Fine needle aspiration cytology of 650 thyroid nodules operated for multinodular goiter: a cyto-histological correlation based on the new Italian cytological classification (SIAPEC 2014). J Biol Regul Homeost Agents 30:1187-1193
Trimboli P, Guidobaldi L, Amendola S et al (2016) Galectin-3 and HBME-1 improve the accuracy of core biopsy in indeterminate thyroid nodules. Endocrine. 52:39-45 https://doi.org/https://doi.org/10.1007/s12020-015-0678-7
Straccia P, Santoro A, Rossi ED et al (2017) Incidence, malignancy rates of diagnoses and cyto-histological correlations in the new Italian Reporting System for Thyroid Cytology: An institutional experience. Cytopathology 28:503-508. https://doi.org/10.1111/cyt.12455
Censi S, Cavedon E, Bertazza L et al (2017) Frequency and Significance of Ras, Tert Promoter, and Braf Mutations in Cytologically Indeterminate Thyroid Nodules: A Monocentric Case Series at a Tertiary-Level Endocrinology Unit. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2017.00273
Medas F, Erdas E, Gordini L et al (2017) Risk of malignancy in thyroid nodules classified as TIR-3A: What therapy? Int J Surg 41:S60-S64. https://doi.org/10.1016/j.ijsu.2017.03.056
Ulisse S, Bosco D, Nardi F et al (2017) Thyroid Imaging Reporting and Data System Score Combined with the New Italian Classification for Thyroid Cytology Improves the Clinical Management of Indeterminate Nodules. Int J Endocrinol. https://doi.org/10.1155/2017/9692304
Lauria A, Maddaloni E, Briganti SI et al (2018) Differences between ATA, AACE/ACE/AME and ACR TI-RADS ultrasound classifications performance in identifying cytological high-risk thyroid nodules. Eur J Endocrinol 178:595-603. https://doi.org/10.1530/EJE-18-0083
Valabrega S, Santolamazza G, Romanelli F et al (2018) Cancer rate of the indeterminate lesions at low or high risk according to italian system for reporting of thyroid FNA. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2018.00371
Trimboli P, Fulciniti F, Merlo E, Barizzi J, Mazzucchelli L, Giovanella L (2018) Histologic Outcome of Indeterminate Thyroid Nodules Classified at Low or High Risk. Endocr Pathol 29:75–79. https://doi.org/10.1007/s12022-018-9517-8
Rezig L, Servadio A, Torregrossa L et al (2018) Diagnosis of post-surgical fine-needle aspiration biopsies of thyroid lesions with indeterminate cytology using HRMAS NMR-based metabolomics. Metabolomics. https://doi.org/10.1007/s11306-018-1437-6
Sparano C, Parenti G, Cilotti A, et al (2018) Clinical impact of the new SIAPEC-IAP classification on the indeterminate category of thyroid nodules. J Endocrinol Invest 42:1-6. https://doi.org/10.1007/s40618-018-0871-7
Rullo E, Minelli G, Bosco D, Nardi F, Ascoli V (2018) Evaluation of the Italian cytological subclassification of thyroid indeterminate nodules into TIR-3A and TIR-3B: a retrospective study of 290 cases with histological correlation from a single institution. J Endocrinol Invest 41:531–538. https://doi.org/10.1007/s40618-017-0763-2
Quaglino F, Marchese V, Mazza E, Gottero C, Lemini R, Taraglio S (2017) When Is Thyroidectomy the Right Choice? Comparison between Fine-Needle Aspiration and Final Histology in a Single Institution Experience. Eur Thyroid J 6:94-100. https://doi.org/10.1159/000452622
Arena S, Benvenga S (2019) The Risk For Malignancy of the Thyroid Nodule is Modulated by Gender, Echotexture, and Intranodular Lymphocytic Thyroiditis. Horm Metab Res. https://doi.org/10.1055/a-0994-0489
Fulciniti F, Cipolletta A, Malzone MG et al (2019) Impact of ultrasonographic features, cytomorphology and mutational testing on malignant and indeterminate thyroid nodules on diagnostic accuracy of fine needle cytology. Clin Endocrinol (Oxf) 91:851-859. https://doi.org/10.1111/cen.14089
Straccia P, Brunelli C, Rossi ED et al (2019) The immunocytochemical expression of VE-1 (BRAF V600E-related) antibody identifies the aggressive variants of papillary thyroid carcinoma on liquid-based cytology. Cytopathology 30:460-467. https://doi.org/10.1111/cyt.12690
Giuliano S, Mirabelli M, Chiefari E et al (2020) Malignancy Analyses of Thyroid Nodules in Patients Subjected to Surgery with Cytological-and Ultrasound-Based Risk Stratification Systems. Endocrines 1:102-118. https://doi.org/10.3390/endocrines1020010
Pastoricchio M, Cubisino A, Lanzaro A et al (2020) Impact of the Italian Society of Anatomic Pathology and Diagnostic Cytology Classification of Thyroid Nodules in the Treatment of Indeterminate Follicular Lesions: Five-Year. Int J Endocrinol. https://doi.org/10.1155/2020/7325260
Sponziello M, Brunelli C, Verrienti A et al (2020) Performance of a dual-component molecular assay in cytologically indeterminate thyroid nodules. Endocrine 68:458-465. https://doi.org/10.1007/s12020-020-02271-y
Piccardo A, Puntoni M, Dezzana M et al (2020) Indeterminate thyroid nodules. The role of 18F-FDG PET/CT in the “era” of ultrasonography risk stratification systems and new thyroid cytology classifications. Endocrine 69:553-561. https://doi.org/10.1007/s12020-020-02239-y
Ianni F, Pascucci D, Paragliola RM et al (2020) Follow-Up or Surgery for Indeterminate Thyroid Nodules: Could the CUT Score Application Be a Support for Decision-Making in the Preoperative Assessment? Thyroid 30:65-71. https://doi.org/10.1089/thy.2018.0649
Cappelli C, Pirola I, Gandossi E et al (2020) Could Serum TSH Levels Predict Malignancy in Euthyroid Patients Affected by Thyroid Nodules with Indeterminate Cytology? Int J Endocrinol. https://doi.org/10.1155/2020/7543930
Capezzone M, Cantara S, Di Sano A et al ( 2021) The Combination of Sonographic Features and the Seven-Gene Panel May be Useful in the Management of Thyroid Nodules With Indeterminate Cytology. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2021.613727
Massa F, Caraci P, Sapino A, De Rosa G, Volante M, Papotti M (2021) Outcome and diagnostic reproducibility of the thyroid cytology “indeterminate categories” SIAPEC/SIE 2014 in a consecutive series of 302 cases. J Endocrinol Invest 44:803-809. https://doi.org/10.1007/s40618-020-01377-4
Pagano L. Costantino E, Bisceglia A et al (2021) Retrospective analysis of the ultrasound features of resected thyroid nodules. Endocrine 72:486-494. https://doi.org/10.1007/s12020-020-02495-y
Possieri C, Locantore P, Salis C et al (2021) Combined molecular and mathematical analysis of long noncoding RNAs expression in fine needle aspiration biopsies as novel tool for early diagnosis of thyroid cancer. Endocrine 72:711-720. https://doi.org/10.1007/s12020-020-02508-w
Javalgi AP, Priyanka P (2021) Evaluation of the Italian Cytological Reporting System and Comparison with Histopathology with Respect to Indeterminate Thyroid Lesions. NJLM 10:14–18. https://doi.org/10.7860/NJLM/2021/46191:2463
Celletti I, Fresilli D, De vito C et al (2021) TIRADS, SRE and SWE in INDETERMINATE thyroid nodule characterization: Which has better diagnostic performance? Radiol Med 126:1189-1200. https://doi.org/10.1007/s11547-021-01349-5
Leni D, Seminati D, Fior D (2021) Diagnostic Performances of the ACR-TIRADS System in Thyroid Nodules Triage: A Prospective Single Center Study. Cancers (Basel). https://doi.org/10.3390/cancers13092230
Poma AM, Macerola E, Basolo A et al (2021) Fine-Needle Aspiration Cytology and Histological Types of Thyroid Cancer in the Elderly: Evaluation of 9070 Patients from a Single Referral Centre. Cancers (Basel) https://doi.org/10.3390/cancers13040907
Fadda G, Basolo F, Bondi A et al (2010) SIAPEC-IAP Italian Consensus Working Group. Cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian Consensus Working Group. Pathologica 102:405-8
Poller DN, Cochand-Priollet B, Trimboli P (2021) Thyroid FNA terminology: The case for a single unified international system for thyroid FNA reporting. Cytopathology 32:714-717. https://doi.org/10.1111/cyt.13017
Valderrabano P, Khazai L, Thompson ZJ et al (2018) Cancer Risk Associated with Nuclear Atypia in Cytologically Indeterminate Thyroid Nodules: A Systematic Review and Meta-Analysis. Thyroid 28:210-219. https://doi.org/10.1089/thy.2017.0419
Crescenzi A, Palermo A, Trimboli P (2021) Cancer prevalence in the subcategories of the indeterminate class III (AUS/FLUS) of the Bethesda system for thyroid cytology: a meta-analysis. J Endocrinol Invest. 44:1343-1351. https://doi.org/10.1007/s40618-021-01526-3
Ali SZ, Cibas E (2017) The Bethesda System for Reporting Thyroid Cytopathology. 2nd edn. Cham, Switzerland: Springer pp 23-26
Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA (2016) Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2: 1023–1029. https://doi.org/10.1001/jamaoncol.2016.0386
M. Bongiovanni, L. Giovanella, F. Romanelli, P. Trimboli. Cytological Diagnoses Associated with Noninvasive Follicular Thyroid Neoplasms with Papillary-Like Nuclear Features According to the Bethesda System for Reporting Thyroid Cytopathology: A Systematic Review and Meta-Analysis. Thyroid. 2019;29:222-228. https://doi.org/10.1089/thy.2018.0394. Epub 2018 Dec 21. PMID: 30426887.
Funding
Open access funding provided by Università della Svizzera italiana.
Author information
Authors and Affiliations
Contributions
PT conceived the study idea. PT and AP conceived the study design. GF, MC, and PT explored the databases and extracted data. PT performed the statistical analysis. PT and AP prepared the draft of the manuscript. All authors read and revised the manuscript, and then approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trimboli, P., Ferrarazzo, G., Cappelli, C. et al. Thyroid Nodules with Indeterminate FNAC According to the Italian Classification System: Prevalence, Rate of Operation, and Impact on Risk of Malignancy. An Updated Systematic Review and Meta-analysis. Endocr Pathol 33, 457–471 (2022). https://doi.org/10.1007/s12022-022-09729-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-022-09729-x