Abstract
Purpose
To assess Strain Ratio (SRE) and Shear Wave Elastography (SWE) accuracy alone and with TIRADS classification, for the risk stratification of indeterminate thyroid nodules.
Materials and methods
128 Patients with 128 indeterminate nodules candidates for thyroidectomy underwent preoperative staging neck ultrasound and were classified according to K-TIRADS score. After TIRADS evaluation, semi-quantitative (SRE) and quantitative (SWE expressed in kPa) elastosonography were performed and relative diagnostic performances, alone and in combination, were compared through ROC curves analysis. In order to maximize the SRE and SWE sensitivity and specificity, their cut-off values were calculated using the Liu test. Bonferroni test was used to evaluate statistically significant differences with a p value < 0.05.
Results
Sensitivity, specificity, PPV and NPV were, respectively, 71.4%, 82.4%, 62.5%, 87.5% for K-TIRADS baseline US, 85.7%, 94.1%, 85.7%, 94.1% for SRE and 57.1%, 79.4%, 53.3%, 81.8% for SWE (kPa expressed). SRE evaluation showed the best diagnostic accuracy compared to the SWE (kPa expressed) (p < 0.05) and to the K-TIRADS (p > 0.05). The association of SRE with conventional ultrasound with K-TIRADS score increased sensitivity (92.9% vs 71.4%) but decreased the specificity than conventional US alone (76.5% vs 82.4%).
Conclusion
Strain Elastosonography can be associated with K-TIRADS US examination in the thyroid nodule characterization with indeterminate cytology; in fact, adding the SRE to K-TIRADS assessment significantly increases its sensitivity and negative predictive value. However, further multicenter studies on larger population are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2018 the "International Agency of Research on Cancer (IARC)” recorded 567,233 new cases of thyroid cancer worldwide with an incidence rate of 3.4/100,000 in men and 11.5/100,000 in women [1].
The growing incidence of thyroid neoplasms is primarily related to the "overdiagnosis" due to the increasingly widespread use of neck imaging techniques such as ultrasound; nevertheless, more than half of the newly diagnosed thyroid cancers in Italy have a low-risk of persistence or recurrence [2].
Recent papers [3] have documented the increase in incidentally thyroid lesions diagnosis that is related to neck ultrasound examinations in 67% [4], to neck CT [5], to MRI in 15% [6] and PET examinations in the remaining 1- 2% of cases [7].
Indeed, ultrasound, being a non-invasive, repeatable over time, widely diffused, and low-cost exam, allows an initial stratification of the risk of malignancy of the thyroid nodule, trying to reduce the number of unnecessary fine-needle aspiration cytology (FNAC). The risk-stratification is based on established criteria in the conventional US (B-mode) imaging: hypoechogenicity, presence of microcalcifications, the anteroposterior diameter larger than transverse diameter (taller-than-wide shape), irregular or lobulated margins [8, 9]. Several risk-stratification systems integrate all these features to group the nodules into discrete risk classes. According to the estimated malignancy risk, the clinician may decide to perform or not FNAC.
FNAC still represents the gold-standard technique for the classification of thyroid nodules, indicating the nature of the lesions with a specificity of 60–98%, but with a highly variable sensitivity (ranging between 54 and 90%) [10].
A thyroid FNAC's main problem is the frequent occurrence of an "inadequate" sampling or an "indeterminate" result.
The "indeterminate" result represents a "gray" diagnostic area, which occurs in 5–20% of cytological reports with the presence of cellular atypia of indeterminate significance (TIR3 category) [11].
TIR3 nodules' management is widely debated, considering that no more than 5–30% of them will be diagnosed as malignant at histological examination. Therefore, there is a growing need for additional diagnostic tools to define the malignancy risk of indeterminate nodules more accurately, reducing the number of surgeries for non-malignant diseases [12].
The adoption of two subcategories based on cyto-morphological features with different malignancy risk and different therapeutic indications were proposed by the Bethesda System [13], the British Thy system [14], and the Italian Consensus of Thyroid Cytology of 2014 (IRSTC: Italian Reporting System for Thyroid Cytology) [15]. In particular, the Italian classification system divided the previous indeterminate category TIR3 into two subcategories: TIR3A, identifying an indeterminate lesion with low risk of malignancy (< 10%) for which a conservative approach is suggested (ultrasound follow-up and FNAC repetition); and TIR3B, identifying a high-risk indeterminate lesion (risk of malignancy 15–30%) [12] for which surgical resection is suggested. The Italian Consensus confirmed these suggestions on the diagnosis and treatment of thyroid carcinoma [16]
The repetition of FNAC for TIR3A nodules is supported by several studies showing a benign reclassification in about 50% of cases [17]. A recent meta-analysis [18] on indeterminate nodules highlighted a malignancy rate of 17% in the TIR3A category and 52% in TIR3B [19], much higher than those expected according to the Italian Consensus of 2014. The management of each cytological class derives from a careful, combined, evaluation of clinical, ultrasound, cytological, and molecular data, if available [20,21,22,23].
Furthermore, recently, diagnostic imaging evaluation has been included additional tools such as elastosonography which evaluates the increased stiffness of thyroid nodules as a sign of malignancy [24,25,26,27,28]. The use of USE methods has been incorporated into international guidelines published by the World Federation for Ultrasound in Medicine and Biology (WFUMB) [29] and the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) [30], that also provide technical details, advantages, and limitations for strain-ratio elastography (SRE) and quantitative 2D ultrasound shear wave elastography (SWE).
The current guidelines [29,30,31] recommend the use of elastosonography as an integrative technique to the B-mode ultrasound evaluation, thanks to its high negative predictive value (false negatives about 3%) [31], but still no specific recommendations have been provided regarding its use for indeterminate thyroid nodules assessment.
Therefore, the present study aimed to evaluate the different diagnostic performance of the two main elastosonography techniques, semi-quantitative (SRE) and quantitative (SWE), in the risk stratification of thyroid nodules with indeterminate cytology in comparison and in addition to the B-mode ultrasound evaluation according to K-TIRADS classification (Table 1) [32].
Materials and methods
Patient enrollment
The present is a prospective study including 128 thyroid nodules in 128 patients (89 women and 39 men) with a mean age of 54.3 years (range 18–82 years) with indeterminate cytology submitted to surgery. The study enrolled patients who referred to Policlinico Umberto I from January 2017 to February 2018. All patients were euthyroid except for five patients who had subclinical hypothyroidism.
The exclusion criteria were: insufficient healthy thyroid parenchyma to perform the elastosonographic evaluation, the presence of large calcifications (> 10% of the nodule size) or large fluid areas (> 50% of the nodule size), and the unavailability of the definitive histological diagnosis (due to surgery performed elsewhere or due to patient refusal to surgery).
All patients had anesthesiology clearance for elective surgery, and acute intercurrent diseases were ruled out.
Cytological evaluation
Ninety-five out of 128 nodules (74%) were classified as TIR3B on cytological examination and were submitted to surgery, according to the current Consensus. The remaining 33 nodules (26%) were classified as TIR3A, and they were referred to surgery for the following reasons: nodule growth during the ultrasound follow-up (9 patients); family history for thyroid neoplasms (6 patients); the concomitant presence of multinodular goiter with initial compressive symptoms (dysphonia and/or dysphagia) (8 patients); size criteria (> 4 cm) (2 patients); previous neck irradiation (2 patients); patient request, due to psychological discomfort for suspected malignancy and/or for aesthetic reasons (6 patients). In all cases, risk factors contraindicating surgery were excluded, and exhaustive and detailed counseling regarding the possible risks related to surgery was offered.
The indication for the cytological examination of TIRADS 3 nodules was based on dimensional criteria (> 1.5 cm) or other patients' risk factors (family history for thyroid cancer, previous neck irradiation, compressive symptoms, high serum calcitonin values).
US evaluation
Patients enrolled underwent a pre-surgical ultrasound evaluation of the neck to plan the most appropriate surgical approach and redefine the site, eco structure, and size of the nodule, its loco-regional extension (with possible thyroid capsule involvement), and the involvement of the cervical lymph nodes. Moreover, each nodule underwent an elastosonographic evaluation to define the degree of hardness, expressed both in semi-quantitative and quantitative terms.
Every patient underwent the pre-surgical ultrasound and elastosonographic evaluation at least 1 month after the cytological sampling, with a mean time of 1.3 months (range 1.1–1.7 months) to avoid possible post-procedural alterations, such as hemorrhagic events, impairing nodule eco-structure and elasticity.
Both the B-mode ultrasound and the elastosonographic examinations with SRE and SWE techniques were performed with Toshiba Aplio 500 or 800 (Osaka, Japan) with a linear 7–15 MHz probe (Figs. 1, 2). All nodules were evaluated by a single operator with at least 10 years of experience in elastosonography and at least 20 years in the ultrasound field within 3–15 days before surgery.
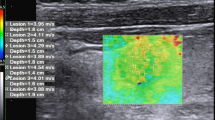
52-year-old patient with a TIR3A thyroid nodule who underwent thyroidectomy for family history for thyroid carcinoma. At histology final diagnosis was Follicular carcinoma. a B-mode US: Mostly hyperechoic, oval shaped, well marginated, solid thyroid nodule, with peripheral hypoechoic margin, without microcalcifications, classified as TIRADS 3; b Semi-quantitative Elastosonography showed a Strain Ratio value of 1.98; c Quantitative Elastosonography showed a value of 16.5 kPa
44-year-old patient with a TIR3B thyroid nodule who underwent a thyroidectomy. At histology final diagnosis was Papillary carcinoma. a B-mode US: Mixed, partially cystic oval shaped and lobulated thyroid nodule, without microcalcifications classified as K-TIRADS 4; b Semi-quantitative Elastosonography showed a Strain Ratio value of 2.47; c Quantitative Elastosonography showed a value of 34.0 kPa
B-mode ultrasound examination was carried out following a predefined protocol aimed at describing for each nodule the following features: the eco-structure (solid or mixed), the echogenicity (hyper-, iso-, hypo-echoic, or markedly hypoechoic), the margins (regular, microlobulated or irregular), calcifications (absent, micro or macrocalcifications) and finally the shape (taller than wide or oval). In order to increase the US sensitivitiy, all nodules were attributed to a risk class following K-TIRADS score. K-TIRADS score was preferred to other TIRADS due to its greater sensitivity in identifying malignant nodules [33] in addition to easy application and high reproducibility [34] and because it is already used and well known by this same working group [35].
Therefore, it is more suited to the widespread use of TIRADS even in non-academic settings, evaluating better the role that elastosonographic techniques could have in clinical routine. On the other hand, K-TIRADS has a low specificity that has not invalidated our study because all nodules were addressed to surgery.
The ultrasound examination was completed with the elastosonographic evaluation with SRE and SWE techniques.
SRE
Slight compressions performed SRE with the probe placed perpendicular to the skin. The examination was prospectively videotaped. The procedure was evaluated in real-time and was considered valid for subsequent evaluations after analyzing the quality indicator. Therefore, during the elastosonographic examination, both the elastogram and the reference B-mode image (twin-view) were displayed on the screen.
Once adequate compression was performed, verified by the complete and exact green color of the quality indicator box, the maximum decompression point was selected, and a ROI was manually positioned on the lesion. A second ROI, of the same size as the previous one, was in the adjacent healthy thyroid tissue at the same depth of the lesion. Then the software calculated the strain value of the tissue with semi-quantitative information (strain ratio SR).
SWE
The quantitative elastosonographic evaluation was carried out using SWE software; the probe was kept stable perpendicular to the lesion, determining the formation of the shear-waves by a mechanical impulse, displayed in real-time to verify their correct propagation (presence of parallel waves propagated in the absence of artifacts or loss of signals). Colorimetric maps were provided, and values expressed in kPa were obtained, positioning a ROI on the thyroid lesion.
All patients underwent total thyroidectomy and the histological analysis was performed according to WHO guidelines. Post-surgical histological diagnosis was considered the gold standard.
K-TIRADS classification, SRE, and SWE values of TIR3 nodules examined and the definitive histological results were recorded in a database for statistical evaluation.
A sample size of 88 was needed to reach a 70% sensitivity and a 80% specificity with a prevalence of 30%. Width of the 95% confidence interval (95% CI) would be at most 20%.
The institutional review board approved the study, and all subjects signed informed consent.
Statistical analysis
Data were collected in a Microsoft Excel database.
All statistical analyses were performed using Stata software (Stata version 15.0, Stata Corporation, College Station, TX, USA).
The comparison of the results was carried out with the histological examination (gold standard). The diagnostic performances of the K-TIRADS score, the SRE, and the SWE were calculated by analyzing the ROC curves. The optimal cut-off values of the continuous variables of Strain and Shear Wave were calculated with the Liu test to maximize their sensitivity and specificity, and their results were used as the basis for data dichotomization. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the ROC curve (AUROC) were calculated. The comparison between the different diagnostic methods and their ROC curves was performed with the Bonferroni test, with results considered statistically significant for p values < 0.05.
Results
Overall, 96 out of 128 indeterminate cytology nodules were included in the analysis (79 TIR3B and 17 TIR3A). Thirty-two nodules were excluded because of the lack of definitive histological diagnosis (28 nodules) and the presence of features not allowing a correct elastosonographic evaluation (4 nodules). In detail, these features were: coarse calcifications (n = 1), excessive fluid components (> 50%; n = 2), and incorrect compression at the SRE documented by the corresponding quality indicator (n = 1).
Ten patients had multinodular goiters but none of them had more than one TIR3 nodule to test.
Thirty-two out of 96 nodules were classified as TIRADS 4 and 5 at the ultrasound examination according to the K-TIRADS system, with an expected high/intermediate risk of malignancy (> 60% and between 15 and 50%, respectively). The remaining 64 nodules were classified as TIRADS 3, with an expected malignancy risk between 3 and 5%.
Final histological examination showed that 68 nodules (71%) were benign (56 follicular hyperplasia, four follicular adenomas, 6 Hürthle cell adenomas, two focal inflammatory lesions) and 28 (29%) were malignant (13 classical papillary carcinomas, 13 follicular variant papillary carcinomas, and two follicular carcinomas).
Moreover, the histological examination showed that 26 (33%) out of 79 TIR3B nodules were malignant; conversely, only 2 (12%) out of 17 TIR3A nodules were found to be malignant.
K-TIRADS assessment detected 20 true positives, 12 false positives, eight false negatives, and 56 true negatives, demonstrating a diagnostic sensitivity of 71.4%, a specificity of 82.4%, a positive predictive value of 62.5%, a negative predictive value of 87.5%, and an AUROC of 0.769.
At the elastosonographic examination, the nodules showed a mean strain ratio of 1.70 (range 0.43–4.91) and a mean SWE value of 31.97 kPa (range 0.92–90). Analyzing the ROC curves of each elastosonographic method, we calculated the optimal cut-off value discriminating the benign and malignant nature of the nodules, and the results were:
-
1.96 for the strain ratio;
-
36.80 kPa for the SWE expressed in kPa.
The semi-quantitative elastosonographic evaluation with Strain Ratio demonstrated high sensitivity (85.7%) and specificity (94.1%), identifying 24 true positives, four false positives, four false negatives, and 64 true negatives, with a positive predictive value of 85.7%, a negative predictive value of 94.1%, and an AUROC of 0.899.
The SWE expressed in kPa, showed a sensitivity of 57.1%, a specificity of 79.4%, a positive predictive value of 53.3%, a negative predictive value of 81.8%, with an AUROC of 0.683.
Overall, the SRE evaluation showed better diagnostic accuracy than SWE expressed in kPa (p < 0.05). The difference between K-TIRADS and SRE was not statistically significant both in terms of sensitivity and specificity.
The addition of the SRE to the SWE assessment did not show a significant increase in diagnostic accuracy. On the other hand, the addition of compressive elastosonography (SRE) to K-TIRADS assessment increased its diagnostic sensitivity (92.9% vs. 71.4%), with a slight reduction in its specificity (specificity of 76.5% vs. 82.4%). There was also an increase in the AUROC (0.847 vs. 0.769) but without statistical significance on the Bonferroni test (p = 0.193).
Statistical analysis stratified by sample size, revealed a reduction in the diagnostic sensitivity of K-TIRADS for nodules > 1 cm (nodule ≤ 1 cm: 85.7%; > 1 cm: 66.7%) and of SWE in kPa (nodule ≤ 1 cm: 71.4%; > 1 cm: 52.4%).
SRE showed the greatest diagnostic accuracy for nodules > 1 cm (sensitivity: 90.5%; specificity: 96.4%, PVP: 90.5%; NPV 96.4%; AUC: 0.935).
The combined evaluation of SRE with K-TIRADS determined a significant increase in diagnostic sensitivity for both nodules ≤ 1 cm (sensitivity 100% vs 85.7%) and nodules > 1 cm (sensitivity 90.5% vs 66.7%).
The SRE + K-TIRADS and SWE kPa + K-TIRADS evaluation showed the maximum diagnostic sensitivity for nodules ≤ 1 cm (100%) with a reduced specificity (SWE kPa + K-TIRADS: 50%; SRE + KTIRADS: 33.3%).
The diagnostic accuracy of the different methods, compared individually and in combination, has been summarized in (Tables 2, 3, 4, 5 and Fig. 3).
Discussion
The clinical management of thyroid nodules with indeterminate cytology still represents a highly controversial topic [36, 37].
Indeed, despite the updates of different cytological classifications, a significant number of nodules (about 20%) is still not adequately characterized on cytological examination [11, 21, 38,39,40] and is referred to surgery for histological diagnosis, leading to patient discomfort, possible complications and costs for the health system.
According to the most recent international guidelines (AACE-ACE-AME 2016) [36], the management of thyroid nodules with indeterminate cytology must be planned based on the cytological sub-classification, clinical data, and ultrasound features. Several potential tools are available (molecular testing [38, 41,42,43], core-needle biopsy, machine-learning approaches [35]), but there is no specific indication of their routine use in this category. Their cost and limited availability reduce their use in clinical practice.
Moreover, despite the availability of different imaging techniques for thyroid nodules, none of them has been proven decisive in discriminating the nature (benign or malignant) of nodules with indeterminate cytology.
Among the emerging imaging techniques in the last decade, US-elastosonography is the most promising [44]; however, its effective contribution in the clinical practice for more accurate characterization of thyroid nodules and especially for indeterminate cytopathology thyroid nodules is still not completely established.
Several studies have shown that a lower elasticity of the thyroid nodule is correlated with a higher incidence of malignancy [45, 46], although this could also be due to other histological features, such as the presence of fibrosis and the expression of galectin- 3 and fibronectin-1 [47].
A meta-analysis reported sensitivity and specificity values of 84% of 90%, respectively, for the SRE in identifying malignant thyroid nodules [48].
Another interesting study showed that the mean strain ratio found in nodules with benign histology was significantly lower than in the malignant group, indicating how the strain ratio index could help distinguish between benign and malignant nodules with a sensitivity of 98.77% and a specificity of 96.30% [49].
In 2015, Liu et al. [50] conducted a study on thyroid nodules lacking suspicious features on ultrasound examination (as in about 15% of papillary thyroid carcinomas), demonstrating the effective contribution of ARFI elastography in identifying malignant nodules, thus confirming the additional value of elastosonographic analysis to B-mode ultrasound.
The recent European (EFSUMB) [30, 31] and World (WFUMB) [29] guidelines have expressed their opinion on the new elastosonographic software, recommending the use in the evaluation of suspected thyroid nodules to be subjected to FNAC [11], thanks to their high positive predictive value (false positives about 3%) [51] and in the follow-up of patients with cytologically benign thyroid nodules. However, they have not recommended their use in evaluating indeterminate thyroid nodules since the available data are still extremely limited and often conflicting.
In this regard, a study from Cantisani et al. [52] evaluating the possible contribution of the SRE in the assessment of nodules with indeterminate cytology found a significant correlation between strain ratio values > 2.05 and malignancy risk, favoring, potentially, a pre-surgical selection with 87.5% sensitivity and 92% specificity.
Furthermore, Samir et al. [53] concluded that the quantitative approach (SWE) is a valuable tool for assessing the preoperative malignancy risk in thyroid nodules with indeterminate cytology, showing a sensitivity of 82% and a specificity of 88% with a 22.3 kPa cut-off.
Conversely, a recent study from Bardet et al. stated that the conventional SWE cut-off values could not discriminate benign and malignant tumors among thyroid nodules with indeterminate cytology [54].
However, currently, which elastosonographic technique has the greatest diagnostic accuracy is yet to be defined.
In this regard, in 2015, a meta-analysis including 54 studies on thyroid nodules (2621 malignant and 7380 benign nodules) undergoing elastosonographic evaluation with a semi-quantitative (SRE) and quantitative (SWE) technique showed that the SRE had better diagnostic sensitivity than SWE with almost overlapping specificity. Indeed, SRE showed sensitivity and specificity values of 83.0% and 81.2%, respectively, both higher than SWE (78.7% and 80.5%, respectively) [55].
More recently (2017), Hu et al. [48] published a meta-analysis including 22 studies, confirming the higher sensitivity of SRE compared to SWE (0.84 vs. 0.79) in thyroid disease, with an AUROC of 0.94 and 0.83, respectively.
The results of the few studies available on indeterminate cytology nodules seem to confirm the semi-quantitative approach (SRE) as a more performing technique than SWE in discriminating the nature of the lesions [56, 57]
In this regard, the study from Gay et al. [58], evaluating the multiparametric assessment of indeterminate thyroid nodule, concluded that the strain ratio was the only parameter significantly correlated to the histological outcomes no significant correlation of the SRE with the SWE.
Beyond the elastosonographic methods, several additional US applications were introduced in the last decade in order to improve the thyroid nodules evaluation and/or reduce its interobserver variability as CEUS and CAD systems. In a recent systematic review, CEUS showed a good sensitivity (73–93%) with a more variable specificity (63–100%) [59] Recently, US-based CAD systems for the differential diagnosis of thyroid nodules have been developed to aid the users in the image interpretation and reduce interobserver variability showing however, lesser sensitivity than expert radiologist with similar specificity [35, 60]
In our study TIR3B observed malignancy rate was slightly higher than expected by the 2014 Italian Consensus and in particular 33% vs 15–30% [16]. This is probably due to the fact that our department is considered a referral center for thyroid pathologies.
In our thyroid nodules’ cohort, we found ten multinodular goiters but none of them had more than one TIR3 nodule to test. The value of thyroid elastography for predicting nodule malignancy in nodular goiters was investigated by several authors. Different elastographic techniques were used all methods has both good sensitivity and specificity for predicting thyroid nodule malignancy [61]
The results of the current study confirm that in the diagnostic imaging algorithm aimed at defining the nature of thyroid nodules with indeterminate cytology, the semi-quantitative elastosonographic evaluation with SR shows the best diagnostic accuracy, both in terms of sensitivity (85.7%) and specificity (94.1%), compared to the SWE (p < 0.05) expressed in kPa (sensitivity: 57.1%; specificity: 79.4%), and to K-TIRADS (not statistically significant) (sensitivity: 71.4%; specificity: 82.4%).
Moreover, the comparison between the different methods shows that SRE evaluation integrated with TI-RADS determines an increase in diagnostic accuracy and, in particular, in its sensitivity (92.9% vs. 71.4%), reducing false negatives but with a slight increase in false positives (specificity of 76.5% vs. 82.4%).
SRE data integrated with K-TIRADS determined an increase in the NPV (NPV K-TIRADS + SRE: 96.3% versus NPV K-TIRADS 87.5%), mainly for nodules ≤ 1 cm, being both the specificity and the NPV 100%.
These results suggest that the combination of classification systems could assess indeterminate nodules malignancy risk with greater accuracy, thus reducing the number of inappropriate surgical interventions.
Therefore, strain elastography, could help to identify nodules that, although lacking suspicious ultrasound features, have a high stiffness value and thus are at risk of malignancy, for which the use of biopsy is the primary indication.
Furthermore, our results reveal how the combined use of the two elastosonographic techniques alone (SRE + SWE) and with K-TIRADS (SRE + SWE + K-TIRADS) does not determine an increase in the diagnostic accuracy of thyroid nodules with indeterminate cytology.
Our study has some limitations. First, this was a relatively small and particular cohort of thyroid nodules, all of which had already been selected for FNA biopsy and surgery by another physician (e.g., endocrinologists, oncologists, general practitioners, clinicians from other fields, pathologists), and the criteria supporting these requests were not completely known. This is reflected in the high malignancy rate. Second, this study was conducted in a single center, and all US examinations were performed by a radiologist with extensive experience in thyroid elastosonographic techniques. This may impact the applicability of our findings to other settings. Finally, our study is lacking an intra-observer and inter-observer agreement evaluation.
In conclusion, the current study results, based on the available technology, demonstrate that the compressive elastosonographic technique with SR semi-quantitative evaluation has greater diagnostic accuracy than the dynamic quantitative technique (shear-wave) in the assessment of indeterminate thyroid nodules. Therefore, it could be considered a useful tool to define the nature of thyroid nodules more accurately, especially if integrated with TIRADS.
Moreover, it would represent a powerful tool in directing patients on a personalized diagnostic and therapeutic path, thus reducing diagnostic thyroidectomies and their relevant consequences (post-procedural complications; health system costs; patient discomfort).
This is a preliminary study, and the results should be validated in a larger population, possibly in multicenter studies, to evaluate the potential bias resulting from inter-operator variability.
Availability of data and material
Yes
Abbreviations
- AUC:
-
Area Under Curve
- AUROC:
-
Area under the Receiver Operating Characteristic Curve
- EFSUMB:
-
European Federation of Ultrasound in Medicine and Biology
- FNAC:
-
Fine-needle aspiration cytology
- MPUS:
-
Multiparametric ultrasound
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region Of Interest
- SRE:
-
Strain ratio elastography
- SWE:
-
Shear wave elastography
- TIRADS:
-
Thyroid Imaging, Reporting, and Data System
- US:
-
Ultrasound
- WFUMB:
-
World Federation of Ultrasound in Medicine and Biology
References
Mp Curado et al (2014) Cancer incidence in five continents. Iarc Scientific Publications. Isnb 978-02-832-2165-4
Grani G, Zatelli MC, Alfò M, Montesano T, Torlontano M, Morelli S, Deandrea M, Antonelli A, Francese C, Ceresini G, Orlandi F, Maniglia CA, Bruno R, Monti S, Santaguida MG, Repaci A, Tallini G, Fugazzola L, Monzani F, Giubbini R, Rossetto R, Mian C, Crescenzi A, Tumino D, Pagano L, Pezzullo L, Lombardi CP, Arvat E, Petrone L, Castagna MG, Spiazzi G, Salvatore D, Meringolo D, Solaroli E, Monari F, Magri F, Triggiani V, Castello R, Piazza C, Rossi R, Ferraro Petrillo U, Filetti S, Durante C (2000) Real-World Performance of the American Thyroid Association Risk estimates in predicting 1-year differentiated thyroid cancer outcomes: a prospective multicenter study of 2000 patients. Thyroid.
Russ G, Leboulleux S, Leenhardt L, Hegedüs L (2014) Thyroid incidentalomas: epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J 3:154–163
Steele SR, Martin MJ, Mullenix PS et al (2005) The significance of incidental thyroid abnormalities identified during carotid duplex ultrasonography. Arch Surg 140(10):981–985
Yoon DY, Chang SK, Choi CS, Yun EJ, Seo YL, Nam ES, Cho SJ, Rho YS, Ahn HY (2008) The prevalence and significance of incidental thyroid nodules identified on computed tomography. J Comput Assist Tomogr 32:810–815
Youserm D, Huang T, Loevner LA et al (1997) Clinical and economic impact of incidental thyroid lesions found with CT and MR. AJNR Am J Neuroradiol 18(8):1423
Sharma SD, Jacques T, Smith S, Watters G (2015) Diagnosis of incidental thyroid nodules on 18F-fluorodeoxyglucose positron emission tomography imaging: are these significant? J Laryngol Otol 129(1):53–56
Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, Hammers LW, Hamper UM, Langer JE, Reading CC, Scoutt LM, Stavros AT (2017) ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol 14(5):587–595
Nabahati M, Moazezi Z, Fartookzadeh S, Mehraeen R, Ghaemian N, Sharbatdaran M (2019) The comparison of accuracy of ultrasonographic features versus ultrasound-guided fine-needle aspiration cytology in diagnosis of malignant thyroid nodules. J Ultrasound 22(3):315–321
Gallo P, D’Amati G (2008) Anatomia patologica, la sistematica, vol.2. UTET
Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW (2012) The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol 56(4):333–339
Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, Friedman L, Kloos RT, LiVolsi VA, Mandel SJ, Raab SS, Rosai J, Steward DL, Walsh PS, Wilde JI, Zeiger MA, Lanman RB, Haugen BR (2012) Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 367(8):705–715
Cibas ES, Ali SZ (2017) The 2017 Bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol 6(6):217–222
Cross P, Chandra A, Giles T, Johnson S, Kocjan G, Poller D, StephensonT (2016) Guidance on the reporting of thyroid cytology specimens. Royal College of Pathologists, London
Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, Palombini L, Papini E, Zini M, Pontecorvi A, Vitti P (2014) Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest 37(6):593–599
Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, Durante C, Elisei R, Fadda G, Frasoldati A, Fugazzola L, Guglielmi R, Lombardi CP, Miccoli P, Papini E, Pellegriti G, Pezzullo L, Pontecorvi A, Salvatori M, Seregni E, Vitti P (2018) Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest 41(7):849–876
Fernandes V, Pereira T, Eloy C (2017) Punção Aspirativa de Nódulos da Tiroide: Vale a Pena Repetir? [Fine-needle Aspiration of Thyroid Nodules: Is it Worth Repeating?]. Acta Med Port 30(6):472–478
Trimboli P, Crescenzi A, Castellana M et al (2019) Italian consensus for the classification and reporting of thyroid cytology: the risk of malignancy between indeterminate lesions at low or high risk. A systematic review and meta-analysis. Endocrine 63(3):430–438
Trimboli P, Crescenzi A, Giovanella L (2018) Performance of Italian Consensus for the Classification and Reporting of Thyroid Cytology (ICCRTC) in discriminating indeterminate lesions at low and high risk of malignancy. A systematic review and meta-analysis. Endocrine.
Grani G, Lamartina L, Ascoli V, Bosco D, Nardi F, D’Ambrosio F, Rubini A, Giacomelli L, Biffoni M, Filetti S, Durante CCV (2016) Ultrasonography scoring systems can rule out malignancy in cytologically indeterminate thyroid nodules. Endocrine 57:256–261
Ulisse S, Bosco D, Nardi F, Nesca A, D’Armiento E, Guglielmino V, De Vito C, Sorrenti S, Pironi D, Tartaglia F, Arcieri S, Catania A, Monti M, Filippini A, Ascoli V (2017) Thyroid imaging reporting and data system score combined with the New Italian classification for thyroid cytology improves the clinical management of tndeterminate nodules. Int J Endocrinol 2017:9692304
Grani G, Sponziello M, Pecce V, Ramundo V, Durante C (2020) Contemporary thyroid nodule evaluation and management. J Clin Endocrinol Metab 105(9):2869–2883
Cozzolino A, Pozza C, Pofi R, Sbardella E, Faggiano A, Isidori AM, Giannetta E, Pernazza A, Rullo E, Ascoli V, Lenzi A, Gianfrilli D (2020) Predictors of malignancy in high-risk indeterminate (TIR3B) cytopathology thyroid nodules. J Endocrinol Invest 43(8):1115–1123
Cantisani V, Lodise P, Di Rocco G et al (2015) Diagnostic accuracy and interobserver agreement of quasistatic ultrasound elastography in the diagnosis of thyroid nodules. Ultraschall Med 36:162–167
Razavi SA, Hadduck TA, Sadigh G et al (2013) Comparative effectiveness of elastographic and B-Mode ultrasound criteria for diagnostic discrimination of thyroid nodules: a meeta-analysis. AJR 200:1317–1326
Ghajarzadeh M, Sodagari F, Shakiba M (2014) Diagnostic accuracy of sonoelastography in detecting malignant thyroid nodules: a systematic review and meta-analysis. AJR Am J Roentgenol 202(4):W379–W389
Nell S, Kist JW, Debray TP et al (2015) Qualitative elastography can replace thyroid nodule fine-needle aspiration in patients with soft thyroid nodules. A systematic review and meta-analysis. Eur J Radiol 84:652–661
Cantisani V, Grazhdani H, Ricci P et al (2014) Q-elastosonography of solid thyroid nodules: assessment of diagnostic efficacy and interobserver variability in a large patient cohort. Eur Radiol 24:143–150
Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, Vinayak S, Xu JM, Dietrich CF (2017) WFUMB guidelines and rrecommendations on the clinical use of Ultrasound Elastography: Part 4. Thyroid Ultrasound Med Biol 43(1):4–26
Bamber J, Cosgrove D, Dietrich CF et al (2013) EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 34:169–184
Săftoiu A, Gilja OH, Sidhu PS, Dietrich CF, Cantisani V, Amy D, Bachmann-Nielsen M, Bob F, Bojunga J, Brock M, Calliada F, Clevert DA, Correas JM, D’Onofrio M, Ewertsen C, Farrokh A, Fodor D, Fusaroli P, Havre RF, Hocke M, Ignee A, Jenssen C, Klauser AS, Kollmann C, Radzina M, Ramnarine KV, Sconfienza LM, Solomon C, Sporea I, Ștefănescu H, Tanter M, Vilmann P (2019) The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic applications: update 2018. Ultraschall Med 40(4):425–453
Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, Lim HK, Moon WJ, Na DG, Park JS, Choi YJ, Hahn SY, Jeon SJ, Jung SL, Kim DW, Kim EK, Kwak JY, Lee CY, Lee HJ, Lee JH, Lee JH, Lee KH, Park SW, Sung JY; Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology (2016) Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 17(3):370–95. https://doi.org/10.3348/kjr.2016.17.3.370. Epub 2016 Apr 14. PMID: 27134526; PMCID: PMC4842857
Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, Maranghi M, Falcone R, Ramundo V, Cantisani V, Filetti S, Durante C (2019) Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “Right” TIRADS. J Clin Endocrinol Metab 104(1):95–102
Grani G, Lamartina L, Cantisani V, Maranghi M, Lucia P, Durante C (2018) Interobserver agreement of various thyroid imaging reporting and data systems. Endocr Connect 7(1):1–7. https://doi.org/10.1530/EC-17-0336. Epub 2017 Dec 1. PMID: 29196301; PMCID: PMC5744624
Fresilli D, Grani G, De Pascali ML, Alagna G, Tassone E, Ramundo V, Ascoli V, Bosco D, Biffoni M, Bononi M, D’Andrea V, Frattaroli F, Giacomelli L, Solskaya Y, Polti G, Pacini P, Guiban O, Gallo Curcio R, Caratozzolo M, Cantisani V (2020) Computer-aided diagnostic system for thyroid nodule sonographic evaluation outperforms the specificity of less experienced examiners. J Ultrasound 23(2):169–174
Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, Paschke R, Valcavi R, Vitti P, AACE, ACE, AME Task Force on Thyroid Nodules (2016) American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules–-2016 update. Endocr Pract 22(5):622–639
Valderrabano P, McIver B (2017) Evaluation and management of indeterminate thyroid nodules: the revolution of risk stratification beyond cytological diagnosis. Cancer Control 24(5):1073274817729231
Sciacchitano S, Lavra L, Ulivieri A et al (2017) Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget 25:49421–49442
Kocjan G, Chandra A, Cross P et al (2009) BSCC Code of Practice—fine needle aspiration cytology. Cytopathology 20:283–296
Valderrabano P, Khazai L, Thompson ZJ et al (2018) Cancer risk associated with nuclear atypia in cytologically indeterminate thyroid nodules: a systematic review and meta-analysis. Thyroid 28(2):210–219
Giusti M, Massa B, Balestra M et al (2017) Retrospective cytological evaluation of indeterminate thyroid nodules according to The British Thyroid Association 2014 Classification And Comparison Of Clinical Evaluation and Outcomes. J Zhejiang Univ Sci B 18:555–566
Ko YS, Hwang TS, Kim JY, Choi YL, Lee SE, Han HS, Kim WS, Kim SK, Park KS (2017) Diagnostic limitation of fine-needle aspiration (FNA) on indeterminate thyroid nodules can be partially overcome by preoperative molecular analysis: assessment of RET/PTC1 rearrangement in BRAF and RAS wild-type routine air-dried FNA specimens. Int J Mol Sci 18(4):806
Decaussin-Petrucci M, Descotes F, Depaepe L, Lapras V, Denier ML, Borson-Chazot F, Lifante JC, Lopez J (2017) Molecular testing of BRAF, RAS and TERT on thyroid FNAs with indeterminate cytology improves diagnostic accuracy. Cytopathology 28(6):482–487
Dietrich CF, Cantisani V (2014) Current status and perspectives of elastography. Eur J Radiol 83(3):403–404
Nn P, Gs P, Manchanda A, Garg A (2017) Diagnostic value of acoustic radiation force impulse quantification in the differentiation of benign and malignant thyroid nodules. Ultrason Imaging 39:326–336
Wang F, Chang C, Gao Y et al (2016) Does shear wave elastography provide additional value in the evaluation of thyroid nodules that are suspicious for malignancy? J Ultrasound Med 35:2397–2404
Rago T, Scutari M, Loiacono V et al (2017) Low elasticity of thyroid nodules on ultrasound elastography is correlated with malignancy, degree of fibrosis, and high expression of Galectin-3 and Fibronectin-1. Thyroid 27:103–110
Hu X, Liu Y, Qian L (2017) Diagnostic potential of real-time elastography (RTE) and shear wave elastography (SWE) to differentiate benign and malignant thyroid nodules: a systematic review and meta-analysis. Medicine (Baltimore) 96(43):e8282
Cakir B, Ersoy R, Fn C et al (2014) Elastosonographic strain index in thyroid nodules with atypia of undetermined significance. J Endocrinol Investig 37:127–133
Liu BJ, Lu F, Xu HX et al (2015) The diagnosis value of acoustic radiation force impulse (ARFI) elastography for thyroid malignancy without highly suspicious features on conventional ultrasound. Int J Clin Exp Med 8:15362–15372
Russ G, Bonnema SJ, Erdogan MF et al (2017) European Thyroid Association Guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J 6:225–237
Cantisani V, Ulisse S, Guaitoli E, De Vito C, Caruso R, Mocini R, D’Andrea V, Ascoli V, Antonaci A, Catalano C, Nardi F, Redler A, Ricci P, De Antoni E, Sorrenti S (2012) Q-elastography in the presurgical diagnosis of thyroid nodules with indeterminate cytology. PLoS ONE 7(11):e50725
Ae S, Dhyani M, Anvari A et al (2015) Shear-wave elastography for the preoperative risk stratification of follicular-patterned lesions of the thyroid: diagnostic accuracy and optimal measurement plane. Radiology 277:565–573
Bardet S, Ciappuccini R, Pellot-Barakat C, Monpeyssen H, Michels Tissier F et al (2017) Shear wave elastography in thyroid nodules with indeterminate cytology: results of a prospective Bicentric Study. Thyroid 27:1441–1449
Tian W, Hao S, Gao B et al (2015) Comparison of diagnostic accuracy of real-time elastography and shear wave elastography in differentiation malignant from benign thyroid nodules. Medicine (Baltimore) 94:e2312
Wojtaszek-Nowicka M, Słowińska-Klencka D, Sporny S et al (2017) The efficiency of elastography in the diagnostics of follicular lesions and nodules with an unequivocal FNA result. Endokrynol Pol 68(6):610–622
Sigrist RMS, Liau J, Kaffas AE et al (2017) Ultrasound elastography: review of techniques and clinical applications. Theranostics 7:1303–1329
Gay S, Schiaffino S, Santamorena G, Massa B, Ansaldo G, Turtulici G, Giusti M (2018) Thyroid team at The Policlinico San Martino Genoa. Role of strain elastography and shear-wave elastography in a multiparametric clinical approach to indeterminate cytology thyroid nodules. Med Sci Monit 24:6273–6279
Trimboli P, Castellana M, Virili C, Havre RF, Bini F, Marinozzi F, D’Ambrosio F, Giorgino F, Giovanella L, Prosch H, Grani G, Radzina M, Cantisani V (2020) Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: a systematic review and meta-analysis using histological standard of reference. Radiol Med 125(4):406–415
Gitto S, Grassi G, De Angelis C, Monaco CG, Sdao S, Sardanelli F, Sconfienza LM, Mauri G (2019) A computer-aided diagnosis system for the assessment and characterization of low-to-high suspicion thyroid nodules on ultrasound. Radiol Med 124(2):118–125
Dighe M, Barr R, Bojunga J, Cantisani V, Chammas MC, Cosgrove D, Cui XW, Dong Y, Fenner F, Radzina M, Vinayak S, Xu JM, Dietrich CF (2017) Thyroid ultrasound: state of the art. Part 2—focal thyroid lesions. Med Ultrason 19(2):195–210. https://doi.org/10.11152/mu-999. PMID: 28440355
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Cantisani reports Lecturer fee from Bracco, Samsung and Toshiba. The other authors have no other conflicts of interest to declare.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Code availability
Strain Ratio Elastography and Shear Wave Elastography by Toshiba Aplio 500 and 800 (Osaka, Japan).
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Celletti, I., Fresilli, D., De Vito, C. et al. TIRADS, SRE and SWE in INDETERMINATE thyroid nodule characterization: Which has better diagnostic performance?. Radiol med 126, 1189–1200 (2021). https://doi.org/10.1007/s11547-021-01349-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-021-01349-5