Abstract
Objectives
This study aims to use ultrasound derived features as biomarkers to assess the malignancy of thyroid nodules in patients who were candidates for FNA according to the ACR TI-RADS guidelines.
Methods
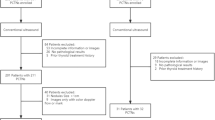
Two hundred and ten patients who met the selection criteria were enrolled in the study and subjected to ultrasound-guided FNA of thyroid nodules. Different radiomics features were extracted from sonographic images, including intensity, shape, and texture feature sets. Least Absolute Shrinkage and Selection Operator (LASSO), Minimum Redundancy Maximum Relevance (MRMR), and Random Forests/Extreme Gradient Boosting Machine (XGBoost) algorithms were used for feature selection and classification of the univariate and multivariate modeling, respectively. Evaluation of models performed using accuracy, sensitivity, specificity, and area under the receiver operating characteristic curve (AUC).
Results
In the univariate analysis, Gray Level Run Length Matrix - Run-Length Non-Uniformity (GLRLM-RLNU) and gray-level zone length matrix - Run-Length Non-Uniformity (GLZLM-GLNU) (both with an AUC of 0.67) were top-performing for predicting nodules malignancy. In the multivariate analysis of the training dataset, the AUC of all combinations of feature selection algorithms and classifiers was 0.99, and the highest sensitivity was for XGBoost classifier and MRMR feature selection algorithms (0.99). Finally, the test dataset was used to evaluate our model in which XGBoost classifier with MRMR and LASSO feature selection algorithms had the highest performance (AUC = 0.95).
Conclusions
Ultrasound-extracted features can be used as non-invasive biomarkers for thyroid nodules’ malignancy prediction.
Key Points
-
Ultrasound imaging features can be used for thyroid nodules’ malignancy prediction.
-
The quantitative features of ultrasound images can be used as non-invasive biomarkers.





Similar content being viewed by others
Abbreviations
- ACR:
-
American College of Radiology
- TI-RADS:
-
Thyroid Imaging, Reporting, and Data System
- ML:
-
Machine Learning
- FNA:
-
Fine-Needle Aspiration
- ROI:
-
Region of Interest
- GLZLM:
-
Grey-Level Zone Length Matrix
- NGLDM:
-
Neighbor Grey-Level Different Matrix
- GLCM:
-
Grey Level Co-Occurrence Matrix
- GLRLM:
-
Grey-Level Run-Length Matrix
- FDR:
-
False Discovery Rate
- ROC:
-
Receiver Operating Characteristic
- SMOTE:
-
Synthetic Minority Oversampling Technique
- LASSO:
-
Least Absolute Shrinkage and Selection Operator
- MRMR:
-
Max-Relevance Min-Redundancy
- RF:
-
Random Forests
- XGBoost:
-
Extreme Gradient Boosting Machine
References
E.A. Azab, A.S. Abdelrahman, M.E.A. Ibrahim, A practical trial to use Thyroid Imaging Reporting and Data System (TI-RADS) in differentiation between benign and malignant thyroid nodules. Egypt. J. Radiol. Nucl. Med. 50, 17 (2019)
S.A. Teefey, W.D. Middleton, C.C. Reading, J.E. Langer, M.D. Beland, M.M. Szabunio, T.S. Desser, Effect of decreasing the ACR TI-RADS point assignment for punctate echogenic foci when they occur in mixed solid and cystic thyroid nodules. Am. J. Roentgenol. 216(2), 479–485 (2021)
P. Aher, A. Juliano, F. Donato, The Role of the ACR TI-RADS Scoring System to Evaluate Solid and Cystic Thyroid Nodules Compared With Those Solid Nodules With or Without Echogenic Foci and Their Cytology Results. J. Diagn. Med. Sonogr. 38(4), 324–329 (2022)
N. Hussain, M.B. Goldstein, M. Zakher, D.S. Katz, T.C. Brandler, S. Islam, G.D. Rothberger, Proportion of Malignancy and Evaluation of Sonographic Features of Thyroid Nodules Classified as Highly Suspicious Using ACR TI-RADS Criteria. J. Ultrasound Med. 42(2), 443–451 (2023)
F.N. Tessler, W.D. Middleton, E.G. Grant, Thyroid imaging reporting and data system (TI-RADS): a user’s guide. Radiology. 287(1), 29–36 (2018)
G. Russ et al. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur. Thyroid J. 6(5), 225–237 (2017)
J. Liang et al. Predicting malignancy in thyroid nodules: radiomics score versus 2017 American College of Radiology Thyroid Imaging, Reporting and Data System. Thyroid 28, 1024–1033 (2018)
T. Liu et al. Comparison of the application of B-mode and strain elastography ultrasound in the estimation of lymph node metastasis of papillary thyroid carcinoma based on a radiomics approach. Int. J. Comput. Assist. Radiol. Surg. 13(10), 1617–1627 (2018)
J.K. Hoang et al. Reduction in thyroid nodule biopsies and improved accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 287(1), 185–193 (2018)
H. Zhao et al. Diagnostic performance of thyroid imaging reporting and data system (TI-RADS) alone and in combination with contrast-enhanced ultrasonography for the characterization of thyroid nodules. Clin. Hemorheol. Microcirc. 72(1), 95–106 (2019)
S. Schenke et al. Diagnostic performance of different thyroid imaging reporting and data systems (Kwak-TIRADS, EU-TIRADS and ACR TI-RADS) for risk stratification of small thyroid nodules (≤10 mm). J. Clin. Med. 9(1), 236 (2020)
S. Rastegar et al. Radiomics for classification of bone mineral loss: a machine learning study. Diagn. Int. Imaging 101(9), 599–610 (2020)
M. Nazari et al. Non-invasive Fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. Radio. Med. 125(8), 754–762 (2020)
I. Shiri et al. Next-generation radiogenomics sequencing for prediction of EGFR and KRAS mutation status in NSCLC patients using multimodal imaging and machine learning algorithms. Mol. Imaging Biol. 22(4), 1132–1148 (2020)
I. Tsougos, A. Vamvakas, C. Kappas, I. Fezoulidis, K. Vassiou, Application of radiomics and decision support systems for breast MR differential diagnosis. Comput. Math. Meth. Med. 2018, 7417126 (2018)
Y.S. Choi, S.S. Ahn, J.H. Chang, S.G. Kang, E.H. Kim, S.H. Kim, R. Jain, S.K. Lee, Machine learning and radiomic phenotyping of lower grade gliomas: improving survival prediction. Eur. Radiol. 30(7), 3834–3842 (2020)
H. Wang et al. Machine learning-based multiparametric MRI radiomics for predicting the aggressiveness of papillary thyroid carcinoma. Eur. J. Radiol. 122, 108755 (2020)
H. Zhou, Y. Jin, L. Dai, M. Zhang, Y. Qiu, K. Wang, J. Tian, J. Zheng, Differential Diagnosis of Benign and Malignant Thyroid Nodules Using Deep Learning Radiomics of Thyroid Ultrasound Images. Eur. J. Radiol. 127, 108992 (2020)
J.K. Hoang, W.D. Middleton, A.E. Farjat, J.E. Langer, C.C. Reading, S.A. Teefey, N. Abinanti, F.J. Boschini, A.J. Bronner, N. Dahiya, B.S. Hertzberg, Reduction in thyroid nodule biopsies and improved accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 287(1), 185–193 (2018)
M. Sollini et al. Texture analysis and machine learning to characterize suspected thyroid nodules and differentiated thyroid cancer: where do we stand? Eur. J. Radiol. 99, 1–8 (2018)
T. Liu et al. Prediction of lymph node metastasis in patients with papillary thyroid carcinoma: a radiomics method based on preoperative ultrasound images. Technol. Cancer Res. Treat. 18, 1533033819831713 (2019)
Y. Wang et al. Comparison study of radiomics and deep learning-based methods for thyroid nodules classification using ultrasound images. IEEE Access 8, 52010–52017 (2020)
G. Bang Jun et al. Benign and malignant thyroid classification using computed tomography radiomics. Proc. SPIE. (2020)
E. Saccenti, H.C. Hoefsloot, A.K. Smilde, J.A. Westerhuis, M.M. Hendriks, Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 10, 361–374 (2014)
J.K. Hoang et al. Comparison of thyroid risk categorization systems and fine-needle aspiration recommendations in a multi-institutional thyroid ultrasound registry. J. Am. Coll Radiol. 18(12), 1605–1613 (2021)
L. Gao et al. Comparison among TIRADS (ACR TI-RADS and KWAK-TI-RADS) and 2015 ATA Guidelines in the diagnostic efficiency of thyroid nodules. Endocrine. 64, 90–96 (2019)
Q. Chen, M. Lin, S.J.F.I.E. Wu, Validating and comparing C-TIRADS, K-TIRADS, and ACR-TIRADS in stratifying the malignancy risk of thyroid nodules. Front. Endocrinol. 13, 899575 (2022)
J.Y. Kwak et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 260(3), 892–899 (2011)
J.H. Shin et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J. Radiol. 17(3), 370–395 (2016)
J. Zhou et al. Superficial Organ and Vascular Ultrasound Group of the Society of Ultrasound in Medicine of the Chinese Medical Association; Chinese Artificial Intelligence Alliance for Thyroid and Breast Ultrasound. 2020 Chinese guidelines for ultrasound malignancy riskstratification of thyroid nodules: the C-TIRADS. Endocrine 70, 256–279 (2020)
E. Horvath et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 94(5), 1748–1751 (2009)
J.M. Fradin, ACR TI‐RADS: an advance in the management of thyroid nodules or Pandora’s box of surveillance? J. Clin. Ultrasound. 48(1), 3–6 (2020)
S.M. Ha et al. Malignancy risk of initially benign thyroid nodules: validation with various Thyroid Imaging Reporting and Data System guidelines. Eur. Radiol. 29, 133–140 (2019)
T. Xu et al. Validation and comparison of three newly-released Thyroid Imaging Reporting and Data Systems for cancer risk determination. Endocrine. 64, 299–307 (2019)
S.J. Yoon et al. Similarities and differences between thyroid imaging reporting and data systems. Am. J. Roentgenol. 213(2), W76–W84 (2019)
S.P. Shayesteh et al. Neo-adjuvant chemoradiotherapy response prediction using MRI based ensemble learning method in rectal cancer patients. Phys. Med. 62, 111–119 (2019)
T.-T. Zhai et al. External validation of nodal failure prediction models including radiomics in head and neck cancer. Oral. Oncol. 112, 105083 (2021)
N. Garau et al. External validation of radiomics‐based predictive models in low‐dose CT screening for early lung cancer diagnosis. Med. Phys. 47(9), 4125–4136 (2020)
Y.W. Park et al. Differentiation of recurrent glioblastoma from radiation necrosis using diffusion radiomics with machine learning model development and external validation. Sci. Rep. 11(1), 1–9 (2021)
I. Shahzadi et al. Analysis of MRI and CT-based radiomics features for personalized treatment in locally advanced rectal cancer and external validation of published radiomics models. Sci. Rep. 12(1), 1–15 (2022)
Y. Sim et al. A radiomics approach for the classification of fibroepithelial lesions on breast ultrasonography. Ultrasound. Med. Biol. 46, 1133–1141 (2020)
K.H. Cha et al. Bladder cancer treatment response assessment in CT using radiomics with deep-learning. Sci. Rep. 7(1), 1–12 (2017)
C. Parmar et al. Machine learning methods for quantitative radiomic biomarkers. Sci. Rep. 5, 13087 (2015)
L. Oakden-Rayner et al. Precision radiologyredicting longevity using feature engineering and deep learning methods in a radiomics framework. Sci. Rep. 7(1), 1–13 (2017)
S.J. Ahn et al. Contrast-enhanced T1-weighted image radiomics of brain metastases may predict EGFR mutation status in primary lung cancer. Sci. Rep. 10(1), 1–9 (2020)
B. Colakoglu, D. Alis, M. Yergin, Diagnostic value of machine learning-based quantitative texture analysis in differentiating benign and malignant thyroid nodules. J. Oncol. 2019, 6328329–6328329 (2019)
B. Zhang et al. Radiomic machine-learning classifiers for prognostic biomarkers of advanced nasopharyngeal carcinoma. Cancer Lett. 403, 21–27 (2017)
E.F. Cleere et al. Radiomic detection of malignancy within thyroid nodules using ultrasonography—a systematic review and meta-analysis. Diagnostics 12(4), 794 (2022)
J. Liang et al. Predicting malignancy in thyroid nodules: radiomics score versus 2017 American College of Radiology Thyroid Imaging, Reporting and Data System. Thyroid 28, 1024–1033 (2018)
I. Shiri et al. Repeatability of radiomic features in magnetic resonance imaging of glioblastoma: test-retest and image registration analyses. Med. Phys. 47, 4265–4280 (2020)
M. Edalat-Javid, I. Shiri, G. Hajianfar, H. Abdollahi, H. Arabi, N. Oveisi, M. Javadian, M. Shamsaei Zafarghandi, H. Malek, A. Bitarafan-Rajabi, M. Oveisi, H. Zaidi, Cardiac SPECT radiomic features repeatability and reproducibility: A multi-scanner phantom study. J. Nucl. Cardiol. 28(6), 2730–2744 (2021)
Funding
This work was supported by the Alborz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.P.S., M.N.; Methodology/study design: S.P.S., E.J., M.A.; Software: M.N., M.K.; Validation: E.J., S.P.S.; Formal analysis: M.N., G.H.; Investigation: M.A., S.P.S.; Resources: M.K., E.J.; Data curation: A.S., S.P.S., M.A.; Writing—original draft: E.J., S.P.S.; Writing—review and editing: M.A., M.K.; Visualization: E.J.; Supervision: S.P.S.; Project administration: S.P.S., A.S.; Funding acquisition: S.P.S., A.S.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
arabi, M., Nazari, M., Salahshour, A. et al. A machine learning-based sonomics for prediction of thyroid nodule malignancies. Endocrine 82, 326–334 (2023). https://doi.org/10.1007/s12020-023-03407-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03407-6