Abstract
Bone marrow mesenchymal stem cell derived exosomes (BMSC-exos) are a crucial means of intercellular communication and can regulate a range of biological processes by reducing inflammation, decreasing apoptosis and promoting tissue repair. The process of intervertebral disc degeneration (IVDD) is accompanied by increased reactive oxygen species (ROS) because of a decrease in the expression of Nrf2, a critical transcription factor that resists excessive ROS. Our study demonstrated that BMSC-exos decreased ROS production by inhibiting Keap1 and promoting Nrf2 expression, attenuating the apoptosis, inflammation, and degeneration of nucelus pulposus (NP) cells. BMSC-exos promoted an increase in Nrf2 and nuclear translocation, while NF-κB expression was downregulated during this process. Additionally, the expression of antioxidative proteins was elevated after treatment with BMSC-exos. In vivo, we found more NP tissue retention in the BMSC-exos-treated group, along with more expression of Nrf2 and antioxidant-related proteins. Our findings demonstrated for the first time that BMSC-exos could restore the down-regulated antioxidant response system in degenerating NP cells by modulating the Keap1/Nrf2 axis. BMSC-exos could be used as an immediate ROS modulator in the treatment of intervertebral disc degeneration.
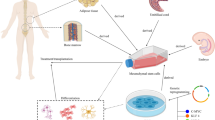
Graphical Abstract
When BMSC-exos were uptaken by NPCs, the expression of Keap1 decreased and this led to increased expression of Nrf2. Nuclear translocation of Nrf2 then promoted the synthesis of antioxidants against ROS and inhibited NF-kB signalling. Cellular inflammation, apoptosis, and ECM-related indicators were further reduced. Together, the process of IVDD was alleviated







Similar content being viewed by others
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Code Availability
Not applicable.
Abbreviations
- BMSC-exos :
-
Bone marrow mesenchymal stem cell derived exosomes
- DAPI :
-
4′,6-diamidino-2-phenylindole
- DHI :
-
Disc height index
- DMEM :
-
Dulbecco’s modified Eagle’s medium
- ECM :
-
Extracellular matrix
- FBS :
-
Foetal bovine serum
- HO-1 :
-
Heme-oxygenase-1
- IVDD :
-
Intervertebral disc degeneration
- Keap1 :
-
Kelch-like ECH-associated protein 1
- NP :
-
Nucleus pulposus
- NQO1 :
-
NADP(H) quinone oxidoreductase 1
- Nrf2 :
-
Transcription factor nuclear factor erythroid-2 related factor 2
- ROS :
-
Reactive oxygen species
- SODs :
-
Superoxide dismutases
- TEM :
-
Transmission electron microscopy
- 8-OHdG :
-
8-Hydroxy-1,2 deoxyguanosine
References
Hoy, D. G., Smith, E., Cross, M., et al. (2014). The global burden of musculoskeletal conditions for 2010: An overview of methods. Annals of the Rheumatic Diseases, 73, 982–989.
Luoma, K., Riihimaki, H., Luukkonen, R., Raininko, R., Viikari-Juntura, E., & Lamminen, A. (2000). Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976), 25, 487–492.
Walker, M. H., & Anderson, D. G. (2004). Molecular basis of intervertebral disc degeneration. The Spine Journal: Official Journal of the North American Spine Society, 4, 158S-166S.
Dowdell, J., Erwin, M., Choma, T., Vaccaro, A., Iatridis, J., & Cho, S. K. (2017). Intervertebral disk degeneration and repair. Neurosurgery, 80, S46–S54.
Song, J., Chen, Z. H., Zheng, C. J., et al. (2020). Exosome-transported circRNA_0000253 competitively Adsorbs MicroRNA-141-5p and increases IDD. Molecular Therapy Nucleic Acids, 21, 1087–1099.
Song, J., Wang, H. L., Song, K. H., et al. (2018). CircularRNA_104670 plays a critical role in intervertebral disc degeneration by functioning as a ceRNA. Experimental & Molecular Medicine, 50, 1–12.
Feng, C., Yang, M., Lan, M., et al. (2017). ROS: Crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxidative Medicine and Cellular Longevity, 2017, 5601593.
Suzuki, S., Fujita, N., Hosogane, N., et al. (2015). Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Research & Therapy, 17, 316.
Poveda, L., Hottiger, M., Boos, N., & Wuertz, K. (2009). Peroxynitrite induces gene expression in intervertebral disc cells. Spine (Phila Pa 1976), 34, 1127–1133.
Bellezza, I., Giambanco, I., Minelli, A., & Donato, R. (2018). Nrf2-Keap1 signaling in oxidative and reductive stress. Biochimica et Biophysica Acta - Molecular Cell Research, 1865, 721–733.
Tonelli, C., Chio, I. I. C., & Tuveson, D. A. (2018). Transcriptional regulation by Nrf2. Antioxidants & Redox Signaling, 29, 1727–1745.
Cullinan, S. B., Gordan, J. D., Jin, J., Harper, J. W., & Diehl, J. A. (2004). The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1 ligase. Molecular and Cellular Biology, 24, 8477–8486.
Keleku-Lukwete, N., Suzuki, M., & Yamamoto, M. (2018). An overview of the Advantages of KEAP1-NRF2 system activation during Inflammatory Disease Treatment. Antioxidants & Redox Signaling, 29, 1746–1755.
Ma, Q. (2013). Role of nrf2 in oxidative stress and toxicity. Annual Review of Pharmacology and Toxicology, 53, 401–426.
Zelko, I. N., Mariani, T. J., & Folz, R. J. (2002). Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine, 33, 337–349.
Lin, J., Chen, J., Zhang, Z., et al. (2019). Luteoloside inhibits IL-1beta-Induced apoptosis and catabolism in nucleus pulposus cells and ameliorates intervertebral disk degeneration. Frontiers in Pharmacology, 10, 868.
Luo, X., Huan, L., Lin, F., et al. (2021). Ulinastatin ameliorates IL-1beta-induced cell dysfunction in human nucleus pulposus cells via Nrf2/NF-kappaB pathway. Oxidative Medicine and Cellular Longevity, 2021, 5558687.
Wang, K., Hu, S., Wang, B., Wang, J., Wang, X., & Xu, C. (2019). Genistein protects intervertebral discs from degeneration via Nrf2-mediated antioxidant defense system: An in vitro and in vivo study. Journal of Cellular Physiology. https://doi.org/10.1002/jcp.28301
Lu, M. C., Zhao, J., Liu, Y. T., et al. (2019). CPUY192018, a potent inhibitor of the Keap1-Nrf2 protein-protein interaction, alleviates renal inflammation in mice by restricting oxidative stress and NF-kappaB activation. Redox Biology, 26, 101266.
Xu, J., Li, H. B., Chen, L., et al. (2019). BML-111 accelerates the resolution of inflammation by modulating the Nrf2/HO-1 and NF-kappaB pathways in rats with ventilator-induced lung injury. International Immunopharmacology, 69, 289–298.
Wang, T., Jian, Z., Baskys, A., et al. (2020). MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials, 257, 120264.
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., & Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9, 654–659.
Wortzel, I., Dror, S., Kenific, C. M., & Lyden, D. (2019). Exosome-mediated metastasis: Communication from a distance. Developmental Cell, 49, 347–360.
Xian, P., Hei, Y., Wang, R., et al. (2019). Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics, 9, 5956–5975.
Shen, K., Jia, Y., Wang, X., et al. (2021). Exosomes from adipose-derived stem cells alleviate the inflammation and oxidative stress via regulating Nrf2/HO-1 axis in macrophages. Free Radical Biology and Medicine, 165, 54–66.
Xia, C., Zeng, Z., Fang, B., et al. (2019). Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radical Biology and Medicine, 143, 1–15.
Issy, A. C., Castania, V., Castania, M., et al. (2013). Experimental model of intervertebral disc degeneration by needle puncture in Wistar rats. Brazilian Journal of Medical and Biological Research, 46, 235–244.
Frapin, L., Clouet, J., Delplace, V., Fusellier, M., Guicheux, J., & Le Visage, C. (2019). Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Advanced Drug Delivery Reviews, 149–150, 49–71.
Sun, J. C., Zheng, B., Sun, R. X., et al. (2019). MiR-499a-5p suppresses apoptosis of human nucleus pulposus cells and degradation of their extracellular matrix by targeting SOX4. Biomedicine & Pharmacotherapy, 113, 108652.
Li, Z., Li, X., Chen, C., Chan, M. T. V., Wu, W. K. K., & Shen, J. (2017). Melatonin inhibits nucleus pulposus (NP) cell proliferation and extracellular matrix (ECM) remodeling via the melatonin membrane receptors mediated PI3K-Akt pathway. Journal of Pineal Research. https://doi.org/10.1111/jpi.12435
Dimozi, A., Mavrogonatou, E., Sklirou, A., & Kletsas, D. (2015). Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. European Cells & Materials, 30, 89–102. discussion 103.
D’Autreaux, B., & Toledano, M. B. (2007). ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology, 8, 813–824.
Madreiter-Sokolowski, C. T., Thomas, C., & Ristow, M. (2020). Interrelation between ROS and ca(2+) in aging and age-related diseases. Redox Biology, 36, 101678.
Yamamoto, M., Kensler, T. W., & Motohashi, H. (2018). The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiological Reviews, 98, 1169–1203.
Labrousse-Arias, D., Martinez-Ruiz, A., & Calzada, M. J. (2017). Hypoxia and redox signaling on extracellular matrix remodeling: From mechanisms to pathological implications. Antioxidants & Redox Signaling, 27, 802–822.
Tang, P., Gu, J. M., Xie, Z. A., et al. (2018). Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radical Biology and Medicine, 120, 368–379.
Cheng, X., Zhang, G., Zhang, L., et al. (2018). Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. Journal of Cellular and Molecular Medicine, 22, 261–276.
Liao, Z., Luo, R., Li, G., et al. (2019). Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics, 9, 4084–4100.
Luo, L., Jian, X., Sun, H., et al. (2021). Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem Cells, 39, 467–481.
Lu, S. C. (2009). Regulation of glutathione synthesis. Molecular Aspects of Medicine, 30, 42–59.
Zhang, H., Davies, K. J. A., & Forman, H. J. (2015). Oxidative stress response and Nrf2 signaling in aging. Free Radical Biology and Medicine, 88, 314–336.
Sakai, D., & Grad, S. (2015). Advancing the cellular and molecular therapy for intervertebral disc disease. Advanced Drug Delivery Reviews, 84, 159–171.
Ortega, N., Behonick, D., Stickens, D., & Werb, Z. (2003). How proteases regulate bone morphogenesis. Annals of the New York Academy of Sciences, 995, 109–116.
Deng, H., Huang, X., & Yuan, L. (2016). Molecular genetics of the COL2A1-related disorders. Mutation Research - Reviews in Mutation Research, 768, 1–13.
Colombier, P., Clouet, J., Hamel, O., Lescaudron, L., & Guicheux, J. (2014). The lumbar intervertebral disc: From embryonic development to degeneration. Joint, Bone, Spine: Revue Du Rhumatisme, 81, 125–129.
Bellezza, I., Tucci, A., Galli, F., et al. (2012). Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. Journal of Nutritional Biochemistry, 23, 1583–1591.
Bao, L., Li, J., Zha, D., et al. (2018). Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-kB pathways. International Immunopharmacology, 54, 245–253.
Wardyn, J. D., Ponsford, A. H., & Sanderson, C. M. (2015). Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochemical Society Transactions, 43, 621–626.
Ahmed, S. M., Luo, L., Namani, A., Wang, X. J., & Tang, X. (2017). Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1863, 585–597.
Zhao, X. J., Yu, H. W., Yang, Y. Z., et al. (2018). Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biology, 18, 124–137.
Singh, A., Venkannagari, S., Oh, K. H., et al. (2016). Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-Deficient NSCLC tumors. ACS Chemical Biology, 11, 3214–3225.
Zhang, H., La Marca, F., Hollister, S. J., Goldstein, S. A., & Lin, C. Y. (2009). Developing consistently reproducible intervertebral disc degeneration at rat caudal spine by using needle puncture. Journal of Neurosurgery. Spine, 10, 522–530.
Mager, S. E. L. A., Breakefield, I., & Wood, X. O. (2013). Extracellular vesicles: Biology and emerging therapeutic opportunities. Nature Reviews. Drug Discovery, 12, 347–357.
Chen, B., Sun, Y., Zhang, J., et al. (2019). Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Research & Therapy, 10, 142.
Xie, L., Chen, Z., Liu, M., et al. (2020). MSC-derived exosomes protect vertebral endplate chondrocytes against apoptosis and calcification via the miR-31-5p/ATF6 Axis. Molecular Therapy Nucleic Acids, 22, 601–614.
Shi, Q. Z., Yu, H. M., Chen, H. M., Liu, M., & Cheng, X. (2021). Exosomes derived from mesenchymal stem cells regulate Treg/Th17 balance in aplastic anemia by transferring miR-23a-3p. Clinical and Experimental Medicine. https://doi.org/10.1007/s10238-021-00701-3
Zimta, A. A., Cenariu, D., & Irimie, A. (2019). The role of Nrf2 activity in cancer development and progression. Cancers (Basel). https://doi.org/10.3390/cancers11111755
Acknowledgements
We thanked Professor Mingxia Fan, the director of Key Laboratory of Magnetic Resonance, East China Normal University, for her help in the MRI of rat tails.
Funding
This work was supported by Shanghai Sailing Program, Shanghai, China (20YF1429900); National Natural Science Foundation of China, China (81972093, 81972109, 82102620, 82172490 and 82272549).
Author information
Authors and Affiliations
Contributions
JS, JYJ, HLW, FZ designed the experiments. GYX, XL, SYL, YXZ, SX and FZ performed the experiments and acquired the data. GYX, XL, XLX and XSM analysed the data. GYX, SYL and FZL supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study was approved by the Ethics Committee of Huashan Hospital, Fudan University (2022-042) and the procedures followed were in accordance with the Helsinki Declaration.
Consent to Participate
The nucleus pulposus tissue were collected after obtaining informed consent from the donors.
Consent for Publication
All authors consented to publish this paper.
Conflict of Interest
The authors declare that they have no conflict of interest in the authorship.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 13.4 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, G., Lu, X., Liu, S. et al. MSC-Derived Exosomes Ameliorate Intervertebral Disc Degeneration By Regulating the Keap1/Nrf2 Axis. Stem Cell Rev and Rep 19, 2465–2480 (2023). https://doi.org/10.1007/s12015-023-10570-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10570-w