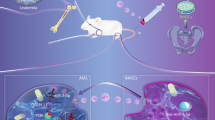
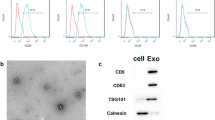
Abstract
Imbalanced Th17/Treg ratio is implicated in the pathogenesis of aplastic anemia. Studies have indicated that bone marrow-derived mesenchymal stem cells-derived exosomes (BMSC-Exo) could correct imbalanced Th17/Treg in aplastic anemia, but the mechanism remains not fully understand. This study was designed to investigate whether BMSC-Exo regulates the Th17/Treg balance in aplastic anemia by transferring miR-23a-3p. Here, miR-23a-3p inhibitor was utilized to knockdown the expression of miR-23a-3p in BMSC-Exo. A co-culture system of CD4+ T cells from aplastic anemia patients and BMSC-Exo was used to explore the effects of BMSC-Exo on the Th17/Treg balance and the underlying mechanism in aplastic anemia. The patients with aplastic anemia exhibited Th17/Treg imbalance favoring the Th17 cells. BMSC-Exo could balance the percentage of Th17 and Treg cells in aplastic anemia, but the effects of BMSC-Exo can be eliminated when miR-23a-3p expression was silenced in BMSCs. IL-6 was a direct target of miR-23a-3p. IL-6 overexpression could abrogate BMSC-Exo-induced balance in Th17/Treg ratio. Overall, BMSC-Exo could balance Th17/Treg ratio in aplastic anemia via suppressing IL-6 expression by transferring miR-23a-3p at least in part. These data indicated miR-23a-3p may be a potential target for the treatment of aplastic anemia. Our study may provide a new idea for the therapy of the disease.




Similar content being viewed by others
Availability of data and materials
The data could be obtained upon request to the corresponding author.
Abbreviations
- AA:
-
Aplastic anemia
- BMSCs:
-
Bone marrow-derived MSCs
- IL:
-
Interleukin
- miRNAs:
-
MicroRNAs
- MSCs:
-
Mesenchymal stem cells
- TGF-β1:
-
Transforming growth factor-β1; Th1: T helper type 1
- Treg:
-
Regulatory T
References
Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207. https://doi.org/10.1111/bjh.13853.
Fattizzo B, Kulasekararaj AG, Hill A, et al. Clinical and morphological predictors of outcome in older aplastic anemia patients treated with eltrombopag. Haematologica. 2019;104:e494–6. https://doi.org/10.3324/haematol.2019.216374.
Boddu PC, Kadia TM. Molecular pathogenesis of acquired aplastic anemia. Eur J Haematol. 2019;102:103–10. https://doi.org/10.1111/ejh.13182.
Zhao J, Chen J, Huang F, et al. Human gingiva tissue-derived MSC ameliorates immune-mediated bone marrow failure of aplastic anemia via suppression of Th1 and Th17 cells and enhancement of CD4+Foxp3+ regulatory T cells differentiation. Am J Transl Res. 2019;11:7627–43.
Li H, Wang L, Pang Y, et al. In patients with chronic aplastic anemia, bone marrow-derived MSCs regulate the Treg/Th17 balance by influencing the Notch/RBP-J/FOXP3/RORγt pathway. Sci Rep. 2017;7:42488. https://doi.org/10.1038/srep42488.
Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules. 2017. https://doi.org/10.3390/molecules22010134.
Zhao L, Chen S, Yang P, Cao H, Li L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10:182. https://doi.org/10.1186/s13287-019-1287-9.
Wang S, Zhu R, Li H, Li J, Han Q, Zhao RC. Mesenchymal stem cells and immune disorders: from basic science to clinical transition. Front Med. 2019;13:138–51. https://doi.org/10.1007/s11684-018-0627-y.
Wang H, Wang Z, Xue M, Liu J, Yan H, Guo Z. Co-transfusion of haplo-identical hematopoietic and mesenchymal stromal cells to treat a patient with severe aplastic. Cytotherapy. 2010;12:563–5. https://doi.org/10.3109/14653241003695059.
Toh WS, Zhang B, Lai RC, Lim SK. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy. 2018;20:1419–26. https://doi.org/10.1016/j.jcyt.2018.09.008.
Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–5. https://doi.org/10.1016/j.biocel.2011.10.005.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. https://doi.org/10.1038/s41556-018-0250-9.
Li Y, Wang F, Guo R, et al. Exosomal sphingosine 1-phosphate secreted by mesenchymal stem cells regulated Treg/Th17 balance in aplastic anemia. IUBMB Life. 2019;71:1284–92. https://doi.org/10.1002/iub.2035.
Hosokawa K, Muranski P, Feng X, et al. Identification of novel microRNA signatures linked to acquired aplastic anemia. Haematologica. 2015;100:1534–45. https://doi.org/10.3324/haematol.2015.126128.
Hosokawa K, Kajigaya S, Feng X, et al. A plasma microRNA signature as a biomarker for acquired aplastic anemia. Haematologica. 2017;102:69–78. https://doi.org/10.3324/haematol.2016.151076.
Giudice V, Banaszak LG, Gutierrez-Rodrigues F, et al. Circulating exosomal microRNAs in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2018;103:1150–9. https://doi.org/10.3324/haematol.2017.182824.
Ferguson SW, Wang J, Lee CJ, et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018;8:1419.
Wu D-M, Wen X, Han X-R, et al. Bone marrow mesenchymal stem cell-derived Exosomal MicroRNA-126-3p inhibits pancreatic cancer development by targeting ADAM9. Mol Ther Nucleic Acids. 2019;16:229–45. https://doi.org/10.1016/j.omtn.2019.02.022.
Guo D, Chen Y, Wang S, et al. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology. 2018;154:132–43. https://doi.org/10.1111/imm.12874.
Kumar BV, Connors TJ, Farber DL. Human T Cell Development, localization, and Function throughout Life. Immunity. 2018;48:202–13. https://doi.org/10.1016/j.immuni.2018.01.007.
Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15:458–69. https://doi.org/10.1038/s41423-018-0004-4.
Naufel AO, Aguiar MCF, Madeira FM, Abreu LG. Treg and Th17 cells in inflammatory periapical disease: a systematic review. Braz Oral Res. 2017;31:e103. https://doi.org/10.1590/1807-3107bor-2017.vol31.0103.
Lu T, Liu Y, Li P, et al. Decreased circulating Th22 and Th17 cells in patients with aplastic anemia. Clin Chim Acta. 2015;450:90–6. https://doi.org/10.1016/j.cca.2015.07.031.
Cheng H, Cao J, Chen W, Qi KM, Li ZY, Xu KL. Th17 Cell Subset levels in peripheral blood of patients with aplastic anemia and their clinical significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:218–24. https://doi.org/10.19746/j.cnki.issn.1009-2137.2020.01.037.
Liu HY, Lin ZH, Liu H, Lu W, Zhang YP. The changes of regulatory T cells and Th17 cells in a novel mouse severe aplastic anemia model. Zhonghua Xue Ye Xue Za Zhi. 2012;33:653–6.
Fattizzo B, Levati G, Cassin R, Barcellini W. Eltrombopag in Immune Thrombocytopenia, Aplastic Anemia, and Myelodysplastic Syndrome: From Megakaryopoiesis to Immunomodulation. Drugs. 2019;79:1305–19. https://doi.org/10.1007/s40265-019-01159-0.
Fattizzo B, Giannotta JA, Barcellini W. Mesenchymal stem cells in aplastic anemia and myelodysplastic syndromes: The “Seed and Soil” crosstalk. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21155438.
Chen QH, Wu F, Liu L, et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther. 2020;11:91. https://doi.org/10.1186/s13287-020-01612-y.
Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71:2020–30. https://doi.org/10.1002/iub.2147.
Quan J, Pan X, Li Y, et al. MiR-23a-3p acts as an oncogene and potential prognostic biomarker by targeting PNRC2 in RCC. Biomed Pharmacother. 2019;110:656–66. https://doi.org/10.1016/j.biopha.2018.11.065.
Ding F, Lai J, Gao Y, et al. NEAT1/miR-23a-3p/KLF3: a novel regulatory axis in melanoma cancer progression. Cancer Cell Int. 2019;19:217. https://doi.org/10.1186/s12935-019-0927-6.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Q-Z Shi designed the study; Q-Z Shi, H-M Yu, H-M Chen, M Liu, and X Cheng conducted the experiments and analyzed the data; Q-Z Shi drafted the paper; and all authors reviewed and approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University.
Informed consent
Informed written consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, Qz., Yu, Hm., Chen, Hm. et al. Exosomes derived from mesenchymal stem cells regulate Treg/Th17 balance in aplastic anemia by transferring miR-23a-3p. Clin Exp Med 21, 429–437 (2021). https://doi.org/10.1007/s10238-021-00701-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-021-00701-3