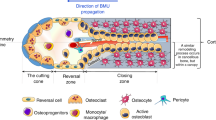
Abstract
As a highly dynamic organ, bone changes during throughout a person’s life. This process is referred to as ‘bone remodeling’ and it involves two stages – a well-balanced osteoclastic bone resorption and an osteoblastic bone formation. Under normal physiological conditions bone remodeling is highly regulated that ensures tight coupling between bone formation and resorption, and its disruption results in a bone metabolic disorder, most commonly osteoporosis. Though osteoporosis is one of the most prevalent skeletal ailments that affect women and men aged over 40 of all races and ethnicities, currently there are few, if any safe and effective therapeutic interventions available. Developing state-of-the-art cellular systems for bone remodeling and osteoporosis can provide important insights into the cellular and molecular mechanisms involved in skeletal homeostasis and advise better therapies for patients. This review describes osteoblastogenesis and osteoclastogenesis as two vital processes for producing mature, active bone cells in the context of interactions between cells and the bone matrix. In addition, it considers current approaches in bone tissue engineering, pointing out cell sources, core factors and matrices used in scientific practice for modeling bone diseases and testing drugs. Finally, it focuses on the challenges that bone regenerative medicine is currently facing.
Graphical Abstract





Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Evans, C. H. (2007). John Hunter and the origins of modern orthopaedic research. Journal of Orthopaedic Research, 25, 556–560. https://doi.org/10.1002/jor.20386
Del Fattore, A., Teti, A., & Rucci, N. (2012). Bone cells and the mechanisms of bone remodelling. Frontiers in Bioscience (Elite Edition), 4, 2302–2321. https://doi.org/10.2741/543
Pfeiffenberger, M., Damerau, A., Lang, A., Buttgereit, F., Hoff, P., & Gaber, T. (2021). Fracture healing research-shift. Towards in Vitro Modeling? Biomedicines, 9. https://doi.org/10.3390/biomedicines9070748
Bolamperti, S., Villa, I., & Rubinacci, A. (2022). Bone remodeling: An operational process ensuring survival and bone mechanical competence.Bone Research, 10. https://doi.org/10.1038/s41413-022-00219-8
de Villiers, T. J., & Goldstein, S. R. (2022) Bone Health 2022: An update. Climacteric, 25, 1–3.https://doi.org/10.1080/13697137.2021.1965408
Khosla, S. (2010). Update in male osteoporosis. Journal of Clinical Endocrinology and Metabolism, 95, 3–10. https://doi.org/10.1210/jc.2009-1740
Shanks, G., Sharma, D., & Mishra, V. (2019). Prevention and treatment of osteoporosis in women. Obstetrics, Gynaecology and Reproductive Medicine, 29, 201–206. https://doi.org/10.1016/j.ogrm.2019.04.001
Rashki Kemmak, A., Rezapour, A., Jahangiri, R., Nikjoo, S., Farabi, H., & Soleimanpour, S. (2020). Economic burden of osteoporosis in the World: A systematic review. Medical Journal of the Islamic Republic of Iran, 34, 154. https://doi.org/10.34171/mjiri.34.154
Domnina, A. P., Krasnova, O. A., Kulakova, K. A., Karelkin, S. Y. V., & Lesnyak, V. V. (2022). N.I.E. Role of G protein-associated membrane receptors in the pathogenesis of osteoporosis. Translational Medicine, 9, 41–61.
Rozenberg, S., Bruyère, O., Bergmann, P., Cavalier, E., Gielen, E., Goemaere, S., Kaufman, J. M., Lapauw, B., Laurent, M. R., & De Schepper, J. (2020). How to manage osteoporosis before the Age of 50. Maturitas, 138, 14–25.https://doi.org/10.1016/j.maturitas.2020.05.004
Lesnyak, O. M., Baranova, I., Belova, K. Y., Gladkova, E. N., Evstigneeva, L. P., Ershova, O. B., Karonova, T. I. K., Yu, A., Nikitinskaya O. A., Skripnikova I. A., Toroptsova N. V., & Aramisova R. M. (2018). Osteoporosis in Russian Federation: Epidemiology, socio-medical and economical aspects.Traumatology and Orthopedics of Russia,24, 155–168.
Liang, B., Burley, G., Lin, S., & Shi, Y. C. (2022). Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cellular & Molecular Biology Letters, 27. https://doi.org/10.1186/s11658-022-00371-3
Miszkiewicz, J. J., Louys, J., & O’Connor, S. (2019). Microanatomical Record of cortical bone remodeling and high vascularity in a Fossil Giant Rat Midshaft Femur. Anatomical Record, 302, 1934–1940. https://doi.org/10.1002/ar.24224
Jilka, R. L. (2013). The relevance of mouse models for investigating age-related bone loss in humans. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68, 1209–1217. https://doi.org/10.1093/gerona/glt046
Owen, R., & Reilly, G. C. (2018). In vitro models of bone remodelling and associated disorders. Frontiers in Bioengineering and Biotechnology, 6, 134. https://doi.org/10.3389/fbioe.2018.00134
Manolagas, S. C. (2000). Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis*. Endocrine Reviews, 21, 115–137. https://doi.org/10.1210/edrv.21.2.0395
Frost, H. M. (1965). A synchronous group of mammallian cells whose in vivo behavior can be studied. Henry Ford Hospital Medical Bulletin, 13, 161–172.
Jilka, R. L. (2003). Biology of the Basic Multicellular Unit and the pathophysiology of osteoporosis. Medical and Pediatric Oncology, 41, 182–185. https://doi.org/10.1002/mpo.10334
Raggatt, L. J., & Partridge, N. C. (2010). Cellular and molecular mechanisms of bone remodeling*. Journal of Biological Chemistry, 285, 25103–25108. https://doi.org/10.1074/jbc.R109.041087
Kenkre, J. S., & Bassett, J. H. D. (2018). The bone remodelling cycle. Annals of Clinical Biochemistry, 55, 308–327. https://doi.org/10.1177/0004563218759371
Kennedy, O. D., Herman, B. C., Laudier, D. M., Majeska, R. J., Sun, H. B., & Schaffler, M. B. (2012). Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone, 50, 1115–1122. https://doi.org/10.1016/j.bone.2012.01.025
Cardoso, L., Herman, B. C., Verborgt, O., Laudier, D., Majeska, R. J., & Schaffler, M. B. (2009). Osteocyte apoptosis controls activation of Intracortical resorption in response to bone fatigue. Journal of Bone and Mineral Research, 24, 597–605. https://doi.org/10.1359/jbmr.081210
Aguirre, J. I., Plotkin, L. I., Stewart, S. A., Weinstein, R. S., Parfitt, A. M., Manolagas, S. C., & Bellido, T. (2006). Osteocyte apoptosis is Induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. Journal of Bone and Mineral Research, 21, 605–615. https://doi.org/10.1359/jbmr.060107
Verborgt, O., Tatton, N. A., Majeska, R. J., & Schaffler, M. B. (2002). Spatial distribution of bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? Journal of Bone and Mineral Research, 17, 907–914. https://doi.org/10.1359/jbmr.2002.17.5.907
Al-Dujaili, S. A., Lau, E., Al-Dujaili, H., Tsang, K., Guenther, A., & You, L. (2011). Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. Journal of Cellular Biochemistry, 112, 2412–2423. https://doi.org/10.1002/jcb.23164
Nakashima, T., Hayashi, M., Fukunaga, T., Kurata, K., Oh-Hora, M., Feng, J. Q., Bonewald, L. F., Kodama, T., Wutz, A., Wagner, E. F., et al. (2011). Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nature Medicine, 17, 1231–1234. https://doi.org/10.1038/nm.2452
Moriishi, T., Fukuyama, R., Ito, M., Miyazaki, T., Maeno, T., Kawai, Y., Komori, H., & Komori, T. (2012). Osteocyte network; a negative regulatory system for bone mass augmented by the induction of rankl in osteoblasts and sost in osteocytes at unloading. PLoS One, 7, e40143.
Cheung, W. Y., Fritton, J. C., Morgan, S. A., Seref-Ferlengez, Z., Basta-Pljakic, J., Thi, M. M., Suadicani, S. O., Spray, D. C., Majeska, R. J., & Schaffler, M. B. (2016). Pannexin-1 and P2 × 7-Receptor are required for apoptotic osteocytes in fatigued bone to trigger RANKL production in neighboring bystander osteocytes. Journal of Bone and Mineral Research, 31, 890–899. https://doi.org/10.1002/jbmr.2740
Ma, Y. L., Cain, R. L., Halladay, D. L., Yang, X., Zeng, Q., Miles, R. R., Chandrasekhar, S., Martin, T. J., & Onyia, J. E. (2001). Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology, 142, 4047–4054. https://doi.org/10.1210/endo.142.9.8356
Li, J., Sarosi, I., Yan, X. Q., Morony, S., Capparelli, C., Tan, H. L., McCabe, S., Elliott, R., Scully, S., Van, G. (2000). RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proceedings of the National Academy of Sciences, 97, 1566–1571. https://doi.org/10.1073/pnas.97.4.1566
Teti, A. (2011). Bone development: overview of bone cells and signaling. Current Osteoporosis Reports, 9, 264–273. https://doi.org/10.1007/s11914-011-0078-8
Andersen, T. L., Abdelgawad, M. E., Kristensen, H. B., Hauge, E. M., Rolighed, L., Bollerslev, J., Kjærsgaard-Andersen, P., & Delaisse, J. M. (2013). Understanding coupling between bone resorption and formation: are reversal cells the missing link? American Journal of Pathology, 183, 235–246. https://doi.org/10.1016/j.ajpath.2013.03.006
Delaisse, J. M., Andersen, T. L., Kristensen, H. B., Jensen, P. R., Andreasen, C. M., & Søe, K. (2020). Re-thinking the bone remodeling cycle mechanism and the origin of bone loss. Bone, 141, 115628. https://doi.org/10.1016/j.bone.2020.115628
Fuller, K., & Chambers, T. J. (1995). Localisation of MRNA for collagenase in osteocytic, bone surface and chondrocytic cells but not osteoclasts. Journal of Cell Science, 108(Pt 6), 2221–2230. https://doi.org/10.1242/jcs.108.6.2221
Delaisse, J. M. (2014). The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. BoneKey Reports, 3. https://doi.org/10.1038/bonekey.2014.56
Lassen, N. E., Andersen, T. L., Pløen, G. G., Søe, K., Hauge, E. M., Harving, S., Eschen, G. E. T., & Delaisse, J. M. (2017). Coupling of bone resorption and formation in real time: new knowledge gained from human haversian BMUs. Journal of Bone and Mineral Research, 32, 1395–1405. https://doi.org/10.1002/jbmr.3091
Blair, H. C., Larrouture, Q. C., Li, Y., Lin, H., Beer-Stoltz, D., Liu, L., Tuan, R. S., Robinson, L. J., Schlesinger, P. H., & Nelson, D. J. (2017). Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Engineering. Part B, Reviews, 23, 268–280. https://doi.org/10.1089/ten.TEB.2016.0454
Sapir-Koren, R., & Livshits, G. (2014). Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption–formation cycles? Osteoporosis International, 25, 2685–2700. https://doi.org/10.1007/s00198-014-2808-0
Winkler, D. G., Sutherland, M. K., Geoghegan, J. C., Yu, C., Hayes, T., Skonier, J. E., Shpektor, D., Jonas, M., Kovacevich, B. R., Staehling-Hampton, K., et al. (2003). Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. Embo Journal, 22, 6267–6276. https://doi.org/10.1093/emboj/cdg599
Franz-Odendaal, T. A., Hall, B. K., & Witten, P. E. (2006). Buried alive: How osteoblasts become osteocytes. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 235, 176–190. https://doi.org/10.1002/dvdy.20603
Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 9, 641–650. https://doi.org/10.1002/jor.1100090504
Andrzejewska, A., Lukomska, B., Janowski, M. C., & Review. (2019). Mesenchymal stem cells: from roots to boost. Stem Cells, 37, 855–864. https://doi.org/10.1002/stem.3016
Huang, S., Jin, M., Su, N., & Chen, L. (2021). New insights on the reparative cells in bone regeneration and repair. Biological Reviews of the Cambridge Philosophical Society, 96, 357–375. https://doi.org/10.1111/brv.12659
Zhang, N., Hu, L., Cao, Z., Liu, X., & Pan, J. (2022). Periosteal skeletal stem cells and their response to bone injury. Frontiers in Cell and Developmental Biology, 10, 812094. https://doi.org/10.3389/fcell.2022.812094
Colnot, C. (2009). Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 24, 274–282. https://doi.org/10.1359/jbmr.081003
Chen, Q., Shou, P., Zheng, C., Jiang, M., Cao, G., Yang, Q., Cao, J., Xie, N., Velletri, T., Zhang, X., et al. (2016). Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death and Differentiation, 23, 1128–1139. https://doi.org/10.1038/cdd.2015.168
Ahmadi, A., Mazloomnejad, R., Kasravi, M., Gholamine, B., Bahrami, S., Sarzaeem, M. M., & Niknejad, H. (2022). Recent advances on small molecules in osteogenic differentiation of stem cells and the underlying signaling pathways. Stem Cell Research & Therapy, 13, 518. https://doi.org/10.1186/s13287-022-03204-4
Xu, X., Zheng, L., Yuan, Q., Zhen, G., Crane, J. L., Zhou, X., & Cao, X. (2018). Transforming growth Factor-β in stem cells and tissue homeostasis. Bone Research, 6, 2. https://doi.org/10.1038/s41413-017-0005-4
Dallas, S. L., Rosser, J. L., Mundy, G. R., & Bonewald, L. F. (2002). Proteolysis of latent transforming growth factor-Beta (TGF-Beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-Beta from bone matrix. Journal of Biological Chemistry, 277, 21352–21360. https://doi.org/10.1074/jbc.M111663200
Tang, Y., Wu, X., Lei, W., Pang, L., Wan, C., Shi, Z., Zhao, L., Nagy, T. R., Peng, X., Hu, J., et al. (2009). TGF-Β1–Induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature Medicine, 15, 757–765. https://doi.org/10.1038/nm.1979
Halloran, D., Durbano, H. W., & Nohe, A. (2020). Bone morphogenetic protein-2 in development and bone homeostasis. Journal of Developmental Biology, 8. https://doi.org/10.3390/jdb8030019
Scarfì, S. (2016). Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World Journal of Stem Cells, 8, 1–12. https://doi.org/10.4252/wjsc.v8.i1.1
Wang, R. N., Green, J., Wang, Z., Deng, Y., Qiao, M., Peabody, M., Zhang, Q., Ye, J., Yan, Z., Denduluri, S., et al. (2014). Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes & Diseases, 1, 87–105. https://doi.org/10.1016/j.gendis.2014.07.005
Xian, L., Wu, X., Pang, L., Lou, M., Rosen, C. J., Qiu, T., Crane, J., Frassica, F., Zhang, L., Rodriguez, J. P., et al. (2012). Matrix IGF-1 maintains bone mass by activation of MTOR in mesenchymal stem cells. Nature Medicine, 18, 1095–1101. https://doi.org/10.1038/nm.2793
Lee, M. N., Hwang, H. S., Oh, S. H., Roshanzadeh, A., Kim, J. W., Song, J. H., Kim, E. S., & Koh, J. T. (2018). Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression. Experimental & Molecular Medicine, 50, 1–16. https://doi.org/10.1038/s12276-018-0170-6
Aquino-Martínez, R., Angelo, A. P., & Pujol, F. V. (2017). Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Research & Therapy, 8, 265. https://doi.org/10.1186/s13287-017-0713-0
Kreja, L., Brenner, R. E., Tautzenberger, A., Liedert, A., Friemert, B., Ehrnthaller, C., Huber-Lang, M., & Ignatius, A. (2010). Non-resorbing osteoclasts induce migration and osteogenic differentiation of mesenchymal stem cells. Journal of Cellular Biochemistry, 109, 347–355. https://doi.org/10.1002/jcb.22406
Yi, S., Kim, J., & Lee, S. Y. (2020). GDNF secreted by pre-osteoclasts induces migration of bone marrow mesenchymal stem cells and stimulates osteogenesis. Bmb Reports, 53, 646–651. https://doi.org/10.5483/BMBRep.2020.53.12.199
Pederson, L., Ruan, M., Westendorf, J. J., Khosla, S., & Oursler, M. J. (2008). Regulation of bone formation by osteoclasts involves Wnt/BMP Signaling and the chemokine sphingosine-1-phosphate. Proceedings of the National Academy of Sciences of the United States of America, 105, 20764–20769. https://doi.org/10.1073/pnas.0805133106
Quint, P., Ruan, M., Pederson, L., Kassem, M., Westendorf, J. J., Khosla, S., & Oursler, M. J. (2013). Sphingosine 1-Phosphate (S1P) receptors 1 and 2 coordinately induce mesenchymal cell migration through S1P activation of complementary kinase pathways. Journal of Biological Chemistry, 288, 5398–5406. https://doi.org/10.1074/jbc.M112.413583
Murakami, S., Nifuji, A., & Noda, M. (1997). Expression of Indian hedgehog in osteoblasts and its posttranscriptional regulation by transforming growth Factor-Β*. Endocrinology, 138, 1972–1978. https://doi.org/10.1210/endo.138.5.5140
Zou, S., Chen, T., Wang, Y., Tian, R., Zhang, L., Song, P., Yang, S., Zhu, Y., Guo, X., Huang, Y., et al. (2014). Mesenchymal stem cells overexpressing Ihh promote bone repair. Journal of Orthopaedic Surgery and Research, 9. https://doi.org/10.1186/s13018-014-0102-7
Maeda, K., Kobayashi, Y., Koide, M., Uehara, S., Okamoto, M., Ishihara, A., Kayama, T., Saito, M., & Marumo, K. (2019). The regulation of bone metabolism and disorders by Wnt signaling. International Journal of Molecular Sciences, 20.
Zhong, Z., Zylstra-Diegel, C. R., Schumacher, C. A., Baker, J. J., Carpenter, A. C., Rao, S., Yao, W., Guan, M., Helms, J. A., Lane, N. E. (2012). Wntless Functions in Mature Osteoblasts to Regulate Bone Mass. Proceedings of the National Academy of Sciences of the United States of America, 109, E2197-204. https://doi.org/10.1073/pnas.1120407109
Lau, K. H. W., Baylink, D. J., Zhou, X. D., Rodriguez, D., Bonewald, L. F., Li, Z., Ruffoni, D., Müller, R., Kesavan, C., & Sheng, M. H. C. (2013). Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. American Journal Of Physiology. Endocrinology And Metabolism, 305, E271–E281. https://doi.org/10.1152/ajpendo.00092.2013
Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., Zanconato, F., Le Digabel, J., Forcato, M., Bicciato, S., et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474, 179–183. https://doi.org/10.1038/nature10137
Chang, Y. C., Wu, J. W., Wang, C. W., & Jang, A. C. C. (2019). Hippo signaling-mediated mechanotransduction in cell movement and cancer metastasis. Frontiers in Molecular Biosciences, 6, 157. https://doi.org/10.3389/fmolb.2019.00157
Yourek, G., McCormick, S. M., Mao, J. J., & Reilly, G. C. (2010). Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regenerative Medicine, 5, 713–724. https://doi.org/10.2217/rme.10.60
Kim, K. M., Choi, Y. J., Hwang, J. H., Kim, A. R., Cho, H. J., Hwang, E. S., Park, J. Y., Lee, S. H., & Hong, J. H. (2014). Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One, 9, e92427. https://doi.org/10.1371/journal.pone.0092427
Arnsdorf, E. J., Tummala, P., Kwon, R. Y., & Jacobs, C. R. (2009). Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. Journal of Cell Science, 122, 546–553. https://doi.org/10.1242/jcs.036293
Arnsdorf, E. J., Tummala, P., & Jacobs, C. R. (2009). Non-canonical wnt signaling and N-Cadherin related Beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One, 4, e5388. https://doi.org/10.1371/journal.pone.0005388
Guillot, P. V., De Bari, C., Dell’Accio, F., Kurata, H., Polak, J., & Fisk, N. M. (2008). Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation, 76, 946–957. https://doi.org/10.1111/j.1432-0436.2008.00279.x
Hwang, J. H., Byun, M. R., Kim, A. R., Kim, K. M., Cho, H. J., Lee, Y. H., Kim, J., Jeong, M. G., Hwang, E. S., & Hong, J. H. (2015). Extracellular matrix stiffness regulates osteogenic differentiation through MAPK activation. PLoS One, 10, 1–16. https://doi.org/10.1371/journal.pone.0135519
Hong, J. H., Hwang, E. S., McManus, M. T., Amsterdam, A., Tian, Y., Kalmukova, R., Mueller, E., Benjamin, T., Spiegelman, B. M., Sharp, P. A., et al. (2005). TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science (80-), 309, 1074–1078. https://doi.org/10.1126/science.1110955
Lee, K. S., Kim, H. J., Li, Q. L., Chi, X. Z., Ueta, C., Komori, T., Wozney, J. M., Kim, E. G., Choi, J. Y., Ryoo, H. M., et al. (2000). Runx2 is a common target of transforming growth factor Beta1 and bone morphogenetic protein 2, and Cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Molecular and Cellular Biology, 20, 8783–8792. https://doi.org/10.1128/MCB.20.23.8783-8792.2000
Galindo, M., Pratap, J., Young, D. W., Hovhannisyan, H., Im, H. J., Choi, J. Y., Lian, J. B., Stein, J. L., Stein, G. S., & van Wijnen, A. J. (2005). The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-Related antiproliferative function in osteoblasts. Journal of Biological Chemistry, 280, 20274–20285. https://doi.org/10.1074/jbc.M413665200
Thomas, D. M., Johnson, S. A., Sims, N. A., Trivett, M. K., Slavin, J. L., Rubin, B. P., Waring, P., McArthur, G. A., Walkley, C. R., Holloway, A. J., et al. (2004). Terminal osteoblast differentiation, mediated by Runx2 and P27KIP1, is disrupted in Osteosarcoma. Journal of Cell Biology, 167, 925–934. https://doi.org/10.1083/jcb.200409187
Wu, H., Whitfield, T. W., Gordon, J. A. R., Dobson, J. R., Tai, P. W. L., van Wijnen, A. J., Stein, J. L., Stein, G. S., & Lian, J. B. (2014). Genomic occupancy of Runx2 with global expression profiling identifies a Novel dimension to control of Osteoblastogenesis. Genome Biology, 15, R52. https://doi.org/10.1186/gb-2014-15-3-r52
Nakashima, K., Zhou, X., Kunkel, G., Zhang, Z., Deng, J. M., Behringer, R. R., & de Crombrugghe, B. (2002). The Novel Zinc Finger-Containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell, 108, 17–29. https://doi.org/10.1016/s0092-8674(01)00622-5
Ortuño, M. J., Susperregui, A. R. G., Artigas, N., Rosa, J. L., & Ventura, F. (2013). Osterix induces Col1a1 gene expression through binding to Sp1 Sites in the bone enhancer and proximal promoter regions. Bone, 52, 548–556. https://doi.org/10.1016/j.bone.2012.11.007
Amarasekara, D. S., Kim, S., & Rho, J. (2021). Regulation of osteoblast differentiation by Cytokine Networks. International Journal of Molecular Sciences, 22, https://doi.org/10.3390/ijms22062851
Na, W., Kang, M. K., Park, S. H., Kim, D. Y., Oh, S. Y., Oh, M. S., Park, S., Kang, I. J., & Kang, Y. H. (2021). Aesculetin accelerates osteoblast differentiation and matrix-vesicle-mediated mineralization. International Journal of Molecular Sciences, 22, https://doi.org/10.3390/ijms222212391
Sun, C., Yuan, H., Wang, L., Wei, X., Williams, L., Krebsbach, P. H., Guan, J. L., & Liu, F. F. A. K. (2016). Promotes osteoblast progenitor cell proliferation and differentiation by enhancing wnt signaling. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 31, 2227–2238. https://doi.org/10.1002/jbmr.2908
Song, L., Liu, M., Ono, N., Bringhurst, F. R., Kronenberg, H. M., & Guo, J. (2012). Loss of Wnt/β-Catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 27, 2344–2358. https://doi.org/10.1002/jbmr.1694
Rutkovskiy, A., Stensløkken, K. O., & Vaage, I. J. (2016). Osteoblast differentiation at a glance. Medical Science Monitor Basic Research, 22, 95–106. https://doi.org/10.12659/msmbr.901142
Kimura, H., Kwan, K. M., Zhang, Z., Deng, J. M., Darnay, B. G., Behringer, R. R., Nakamura, T., de Crombrugghe, B., & Akiyama, H. (2008). Cthrc1 is a positive Regulator of Osteoblastic bone formation. PLoS One, 3, e3174. https://doi.org/10.1371/journal.pone.0003174
Takeshita, S., Fumoto, T., Matsuoka, K., Park, K., Aburatani, H., Kato, S., Ito, M., & Ikeda, K. (2013). Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. Journal of Clinical Investigation, 123, 3914–3924. https://doi.org/10.1172/JCI69493
Roelofsen, T., Akkers, R., Beumer, W., Apotheker, M., Steeghs, I., van de Ven, J., Gelderblom, C., Garritsen, A., & Dechering, K. (2008). Sphingosine-1-Phosphate Acts as a developmental stage specific inhibitor of platelet-derived growth Factor-Induced Chemotaxis of osteoblasts. Journal of Cellular Biochemistry, 105, 1128–1138. https://doi.org/10.1002/jcb.21915
Lotinun, S., Kiviranta, R., Matsubara, T., Alzate, J. A., Neff, L., Lüth, A., Koskivirta, I., Kleuser, B., Vacher, J., Vuorio, E., et al. (2013). Osteoclast-specific cathepsin K deletion stimulates S1P-Dependent bone formation. Journal of Clinical Investigation, 123, 666–681. https://doi.org/10.1172/JCI64840
Alfaro, D., Rodríguez-Sosa, M. R., Zapata, A. G., Arthur, A., & Gronthos, S. (2020). Eph/Ephrin Signaling and Biology of Mesenchymal Stromal/Stem cells. Journal of Clinical Medicine, 9, 598612. https://doi.org/10.3390/jcm9020310
Arthur, A., & Gronthos, S. (2021). Eph-Ephrin Signaling mediates Cross-Talk within the bone microenvironment. Frontiers in Cell and Developmental Biology, 9, 598612. https://doi.org/10.3389/fcell.2021.598612
Zhao, C., Irie, N., Takada, Y., Shimoda, K., Miyamoto, T., Nishiwaki, T., Suda, T., & Matsuo, K. (2006). Bidirectional EphrinB2-EphB4 signaling controls bone homeostasis. Cell Metabolism, 4, 111–121. https://doi.org/10.1016/j.cmet.2006.05.012
Furuya, M., Kikuta, J., Fujimori, S., Seno, S., Maeda, H., Shirazaki, M., Uenaka, M., Mizuno, H., Iwamoto, Y., Morimoto, A., et al. (2018). Direct cell-cell contact between mature osteoblasts and osteoclasts dynamically controls their functions in vivo. Nature Communications, 9, 300. https://doi.org/10.1038/s41467-017-02541-w
Vrahnas, C., & Sims, N. A. (2015). EphrinB2 signalling in osteoblast differentiation, bone formation and endochondral ossification. Current Molecular Biology Reports, 1, 148–156. https://doi.org/10.1007/s40610-015-0024-0
Yu, S., Franceschi, R. T., Luo, M., Fan, J., Jiang, D., Cao, H., Kwon, T. G., Lai, Y., Zhang, J., Patrene, K., et al. (2009). Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PLoS One, 4, e7583.
Lawal, R. A., Zhou, X., Batey, K., Hoffman, C. M., Georger, M. A., Radtke, F., Hilton, M. J., Xing, L., Frisch, B. J., & Calvi, L. M. (2017). The notch ligand Jagged1 regulates the Osteoblastic lineage by maintaining the Osteoprogenitor Pool. Journal of Bone and Mineral Research, 32, 1320–1331. https://doi.org/10.1002/jbmr.3106
Youngstrom, D. W., Dishowitz, M. I., Bales, C. B., Carr, E., Mutyaba, P. L., Kozloff, K. M., Shitaye, H., Hankenson, K. D., & Loomes, K. M. (2016). Jagged1 expression by osteoblast-lineage cells regulates trabecular bone Mass and Periosteal Expansion in mice. Bone, 91, 64–74. https://doi.org/10.1016/j.bone.2016.07.006
Ashley, J. W., Ahn, J., & Hankenson, K. D. (2015). Notch Signaling promotes osteoclast maturation and resorptive activity. Journal of Cellular Biochemistry, 116, 2598–2609. https://doi.org/10.1002/jcb.25205
Calvi, L. M., Adams, G. B., Weibrecht, K. W., Weber, J. M., Olson, D. P., Knight, M. C., Martin, R. P., Schipani, E., Divieti, P., Bringhurst, F. R., et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature, 425, 841–846. https://doi.org/10.1038/nature02040
Park, D., Hoggatt, J., Ferraro, F., & Scadden, D. T. (2013). Chapter 7 - The Skeletal Stem Cell. In Marcus, R., Feldman, D., Dempster, D. W., Luckey, M., & Cauley, J. A. (Eds) Osteoporosis (4th Edition, pp. 127–147). Academic, ISBN 978-0-12-415853-5.
Wein, M. N. (2017). Bone lining cells: Normal physiology and role in response to anabolic osteoporosis treatments. Current Molecular Biology Reports, 3, 79–84. https://doi.org/10.1007/s40610-017-0062-x
Jilka, R. L., Weinstein, R. S., Bellido, T., Parfitt, A. M., & Manolagas, S. C. (1998). Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 13, 793–802. https://doi.org/10.1359/jbmr.1998.13.5.793
Chen, H., Senda, T., & Kubo, K. (2015). The Osteocyte Plays multiple roles in bone remodeling and Mineral Homeostasis. Medical Molecular Morphology, 48, 61–68. https://doi.org/10.1007/s00795-015-0099-y
Hardy, E., & Fernandez-Patron, C. (2020). Destroy to rebuild: The connection between bone tissue remodeling and Matrix Metalloproteinases. Frontiers in Physiology, 11, https://doi.org/10.3389/fphys.2020.00047
Matic, I., Matthews, B. G., Wang, X., Dyment, N. A., Worthley, D. L., Rowe, D. W., Grcevic, D., & Kalajzic, I. (2016). Quiescent bone lining cells are a major source of osteoblasts during Adulthood. Stem Cells, 34, 2930–2942. https://doi.org/10.1002/stem.2474
Håkelien, A. M., Bryne, J. C., Harstad, K. G., Lorenz, S., Paulsen, J., Sun, J., Mikkelsen, T. S., Myklebost, O., & Meza-Zepeda, L. A. (2014). The Regulatory Landscape of osteogenic differentiation. Stem Cells, 32, 2780–2793. https://doi.org/10.1002/stem.1759
Kostina, D., Lobov, A., Klausen, P., Karelkin, V., Tikhilov, R., Bozhkova, S., Sereda, A., Ryumina, N., Enukashvily, N., & Malashicheva, A. (2022). Isolation of human osteoblast cells capable for mineralization and synthetizing bone-related proteins in vitro from adult bone. Cells, 11(21), 3356. https://doi.org/10.3390/cells11213356
Patel, C., Shi, L., Whitesides, J. F., Foster, B. M., Fajardo, R. J., Quillen, E. E., & Kerr, B. A. (2022). A New Method of Bone Stromal Cell characterization by Flow Cytometry. Current Protocols, 2, e400. https://doi.org/10.1002/cpz1.400
Byun, M. R., Hwang, J. H., Kim, A. R., Kim, K. M., Hwang, E. S., Yaffe, M. B., & Hong, J. H. (2014). Canonical wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death and Differentiation, 21, 854–863. https://doi.org/10.1038/cdd.2014.8
Twine, N. A., Chen, L., Pang, C. N., Wilkins, M. R., & Kassem, M. (2014). Identification of differentiation-stage specific markers that define the ex vivo osteoblastic phenotype. Bone, 67, 23–32. https://doi.org/10.1016/j.bone.2014.06.027
Aasebø, E., Brenner, A. K., Hernandez-Valladares, M., Birkeland, E., Berven, F. S., Selheim, F., & Bruserud, Ø. (2021). Proteomic comparison of bone marrow derived osteoblasts and mesenchymal stem cells. International Journal of Molecular Sciences, 22. https://doi.org/10.3390/ijms22115665
Liu, H., Peng, F., Liu, Z., Jiang, F., Li, L., Gao, S., Wang, G., Song, J., Ruan, E., Shao, Z., et al. (2017). CYR61/CCN1 stimulates proliferation and differentiation of osteoblasts in Vitro and contributes to bone remodeling in vivo in Myeloma Bone Disease. International Journal of Oncology, 50, 631–639. https://doi.org/10.3892/ijo.2016.3815
Nistala, H., Lee-Arteaga, S., Smaldone, S., Siciliano, G., Carta, L., Ono, R., Sengle, G., Arteaga-Solis, E., Levasseur, R., Ducy, P., et al. (2010). Fibrillin-1 and – 2 differentially modulate endogenous TGF-β and BMP bioavailability during bone formation. Journal of Cell Biology, 190, 1107–1121. https://doi.org/10.1083/jcb.201003089
Gao, M. M., Su, Q. N., Liang, T. Z., Ma, J. X., Liang, T. Z., Stoddart, M. J., Richards, R. G., Zhou, Z. Y., & Zou, N. X. (2018). Transcriptional activation of ENPP1 by Osterix in osteoblasts and osteocytes. European Cells & Materials, 36, 1–14. https://doi.org/10.22203/eCM.v036a01
Liu, F., Malaval, L., & Aubin, J. E. (1997). The mature osteoblast phenotype is characterized by extensive plasticity. Experimental Cell Research, 232, 97–105. https://doi.org/10.1006/excr.1997.3501
Yang, X., Matsuda, K., Bialek, P., Jacquot, S., Masuoka, H. C., Schinke, T., Li, L., Brancorsini, S., Sassone-Corsi, P., Townes, T. M., et al. (2004). ATF4 is a substrate of RSK2 and an essential Regulator of osteoblast Biology; implication for Coffin-Lowry Syndrome. Cell, 117, 387–398. https://doi.org/10.1016/s0092-8674(04)00344-7
Zohar, R., Cheifetz, S., McCulloch, C. A., & Sodek, J. (1998). Analysis of intracellular osteopontin as a marker of Osteoblastic Cell differentiation and Mesenchymal Cell Migration. European Journal of Oral Sciences, 106(Suppl 1), 401–407. https://doi.org/10.1111/j.1600-0722.1998.tb02206.x
Yu, H., de Vos, P., & Ren, Y. (2011). Overexpression of osteoprotegerin promotes preosteoblast differentiation to mature osteoblasts. Angle Orthodontist, 81, 100–106. https://doi.org/10.2319/050210-238.1
Gordon, J. A. R., Tye, C. E., Sampaio, A. V., Underhill, T. M., Hunter, G. K., & Goldberg, H. A. (2007). Bone sialoprotein expression enhances osteoblast differentiation and Matrix Mineralization in Vitro. Bone, 41, 462–473. https://doi.org/10.1016/j.bone.2007.04.191
Supronowicz, P., Gill, E., Trujillo, A., Thula, T., Zhukauskas, R., Ramos, T., & Cobb, R. (2010). Human adipose-derived side Population Stem cells cultured on demineralized bone matrix for bone tissue Engineering. Tissue Engineering Part A, 17, 789–798. https://doi.org/10.1089/ten.TEA.2010.0357
Hikita, A., Yana, I., Wakeyama, H., Nakamura, M., Kadono, Y., Oshima, Y., Nakamura, K., Seiki, M., & Tanaka, S. (2006). Negative regulation of Osteoclastogenesis by Ectodomain shedding of receptor activator of NF-ΚB Ligand*. Journal of Biological Chemistry, 281, 36846–36855. https://doi.org/10.1074/jbc.M606656200
Akiva, A., Melke, J., Ansari, S., Liv, N., van der Meijden, R., van Erp, M., Zhao, F., Stout, M., Nijhuis, W. H., de Heus, C., et al. (2021). An Organoid for Woven Bone. Advanced Functional Materials, 31, 2010524. https://doi.org/10.1002/adfm.202010524
Kitahama, S., Gibson, M. A., Hatzinikolas, G., Hay, S., Kuliwaba, J. L., Evdokiou, A., Atkins, G. J., & Findlay, D. M. (2000). Expression of fibrillins and other Microfibril-Associated Proteins in Human Bone and osteoblast-like cells. Bone, 27, 61–67. https://doi.org/10.1016/S8756-3282(00)00292-1
Miao, D., & Scutt, A. (2002). Histochemical localization of Alkaline phosphatase activity in decalcified bone and cartilage. Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society, 50, 333–340. https://doi.org/10.1177/002215540205000305
Buenzli, P. R., & Sims, N. A. (2015). Quantifying the Osteocyte Network in the human skeleton. Bone, 75, 144–150. https://doi.org/10.1016/j.bone.2015.02.016
Tresguerres, F. G. F., Torres, J., López-Quiles, J., Hernández, G., Vega, J. A., & Tresguerres, I. F. (2020). The osteocyte: A multifunctional cell within the bone. Annals of Anatomy = Anatomischer Anzeiger: Official Organ of the Anatomische Gesellschaft, 227, 151422. https://doi.org/10.1016/j.aanat.2019.151422
Bonewald, L. F. (2011). The amazing osteocyte. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 26, 229–238. https://doi.org/10.1002/jbmr.320
Prideaux, M., Loveridge, N., Pitsillides, A. A., & Farquharson, C. (2012). Extracellular matrix mineralization promotes E11/Gp38 glycoprotein expression and drives osteocytic differentiation. PLoS One, 7, e36786. https://doi.org/10.1371/journal.pone.0036786
Hirao, M., Hashimoto, J., Yamasaki, N., Ando, W., Tsuboi, H., Myoui, A., & Yoshikawa, H. (2007). Oxygen tension is an important mediator of the Transformation of osteoblasts to Osteocytes. Journal of Bone and Mineral Metabolism, 25, 266–276. https://doi.org/10.1007/s00774-007-0765-9
Mullen, C. A., Haugh, M. G., Schaffler, M. B., Majeska, R. J., & McNamara, L. M. (2013). Osteocyte differentiation is regulated by Extracellular Matrix stiffness and intercellular separation. Journal of the Mechanical Behavior of Biomedical Materials, 28, 183–194. https://doi.org/10.1016/j.jmbbm.2013.06.013
Torreggiani, E., Matthews, B. G., Pejda, S., Matic, I., Horowitz, M. C., Grcevic, D., & Kalajzic, I. (2013). Preosteocytes/Osteocytes have the potential to dedifferentiate becoming a source of osteoblasts. PLoS One, 8, e75204. https://doi.org/10.1371/journal.pone.0075204
Palumbo, C., Palazzini, S., Zaffe, D., & Marotti, G. (1990). Osteocyte differentiation in the tibia of newborn rabbit: An ultrastructural study of the formation of cytoplasmic processes. Acta Anatomica (Basel), 137, 350–358. https://doi.org/10.1159/000146907
Wee, N. K., Sims, N. A., & Morello, R. (2021). The Osteocyte Transcriptome: Discovering messages buried within bone. Current Osteoporosis Reports, 19, 604–615. https://doi.org/10.1007/s11914-021-00708-5
van Bezooijen, R. L., Roelen, B. A. J., Visser, A., van der Wee-Pals, L., de Wilt, E., Karperien, M., Hamersma, H., Papapoulos, S. E., ten Dijke, P., & Löwik, C. W. G. M. (2004). Sclerostin is an osteocyte-expressed negative Regulator of bone formation, but not a classical BMP antagonist. Journal of Experimental Medicine, 199, 805–814. https://doi.org/10.1084/jem.20031454
Hua, R., Gu, S., & Jiang, J. X. (2022). Connexin 43 Hemichannels regulate osteoblast to Osteocyte differentiation. Frontiers in Cell and Developmental Biology, 10, 892229. https://doi.org/10.3389/fcell.2022.892229
Staines, K. A., Prideaux, M., Allen, S., Buttle, D. J., Pitsillides, A. A., & Farquharson, C. (2016). E11/Podoplanin protein stabilization through inhibition of the Proteasome promotes osteocyte differentiation in Murine in Vitro Models. Journal of Cellular Physiology, 231, 1392–1404. https://doi.org/10.1002/jcp.25282
McNamara, L. M., Majeska, R. J., Weinbaum, S., Friedrich, V., & Schaffler, M. B. (2009). Attachment of Osteocyte cell processes to the bone matrix. Anatomical Record (Hoboken), 292, 355–363. https://doi.org/10.1002/ar.20869
Klein-Nulend, J., van der Plas, A., Semeins, C. M., Ajubi, N. E., Frangos, J. A., Nijweide, P. J., & Burger, E. H. (1995). Sensitivity of Osteocytes to Biomechanical stress in Vitro. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 9, 441–445. https://doi.org/10.1096/fasebj.9.5.7896017
Burger, E. H., & Klein-Nulend, J. (1999). Mechanotransduction in Bone–Role of the Lacuno-Canalicular Network. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 13(Suppl), S101-12.
Vahidi, G., Rux, C., Sherk, V. D., & Heveran, C. M. (2021). Lacunar-Canalicular bone remodeling: Impacts on Bone Quality and Tools for Assessment. Bone, 143, 115663. https://doi.org/10.1016/j.bone.2020.115663
Holmbeck, K., Bianco, P., Pidoux, I., Inoue, S., Billinghurst, R. C., Wu, W., Chrysovergis, K., Yamada, S., Birkedal-Hansen, H., & Poole, A. R. (2005). The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. Journal of Cell Science, 118, 147–156. https://doi.org/10.1242/jcs.01581
Staines, K. A., Javaheri, B., Hohenstein, P., Fleming, R., Ikpegbu, E., Unger, E., Hopkinson, M., Buttle, D. J., Pitsillides, A. A., & Farquharson, C. (2017). Hypomorphic conditional deletion of E11/Podoplanin reveals a role in Osteocyte Dendrite Elongation. Journal of Cellular Physiology, 232, 3006–3019. https://doi.org/10.1002/jcp.25999
Staines, K. A., Ikpegbu, E., Törnqvist, A. E., Dillon, S., Javaheri, B., Amin, A. K., Clements, D. N., Buttle, D. J., Pitsillides, A. A., & Farquharson, C. (2019). Conditional deletion of E11/Podoplanin in bone protects against Load-Induced Osteoarthritis. Bmc Musculoskeletal Disorders, 20, 344. https://doi.org/10.1186/s12891-019-2731-9
Zhang, K., Barragan-Adjemian, C., Ye, L., Kotha, S., Dallas, M., Lu, Y., Zhao, S., Harris, M., Harris, S. E., Feng, J. Q., et al. (2006). E11/Gp38 selective expression in Osteocytes: Regulation by mechanical strain and role in Dendrite Elongation. Molecular and Cellular Biology, 26, 4539–4552. https://doi.org/10.1128/MCB.02120-05
Plotkin, L. I., & Bellido, T. (2013). Beyond gap junctions: Connexin43 and bone cell signaling. Bone, 52, 157–166. https://doi.org/10.1016/j.bone.2012.09.030
Yellowley, C. E., Li, Z., Zhou, Z., Jacobs, C. R., & Donahue, H. J. (2000). Functional gap junctions between Osteocytic and Osteoblastic cells. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 15, 209–217. https://doi.org/10.1359/jbmr.2000.15.2.209
Kamioka, H., Honjo, T., Takano-Yamamoto, T. A., & Three-Dimensional. (2001). Distribution of osteocyte processes revealed by the combination of Confocal Laser scanning Microscopy and Differential Interference contrast Microscopy. Bone, 28, 145–149. https://doi.org/10.1016/s8756-3282(00)00421-x
Riquelme, M. A., Cardenas, E. R., Xu, H., & Jiang, J. X. (2020). The role of Connexin channels in the response of mechanical loading and unloading of bone. International Journal of Molecular Sciences, 21, https://doi.org/10.3390/ijms21031146
Cheng, B., Zhao, S., Luo, J., Sprague, E., Bonewald, L. F., & Jiang, J. X. (2001). Expression of functional gap Junctions and Regulation by Fluid Flow in Osteocyte-Like MLO-Y4 cells. Journal of Bone and Mineral Research, 16, 249–259. https://doi.org/10.1359/jbmr.2001.16.2.249
Siller-Jackson, A. J., Burra, S., Gu, S., Xia, X., Bonewald, L. F., Sprague, E., & Jiang, J. X. (2008). Adaptation of Connexin 43-Hemichannel prostaglandin release to mechanical loading. Journal of Biological Chemistry, 283, 26374–26382. https://doi.org/10.1074/jbc.M803136200
Batra, N., Burra, S., Siller-Jackson, A. J., Gu, S., Xia, X., Weber, G. F., DeSimone, D., Bonewald, L. F., Lafer, E. M., Sprague, E. (2012). Mechanical Stress-Activated Integrin Α5β1 Induces Opening of Connexin 43 Hemichannels. Proceedings of the National Academy of Sciences of the United States of America, 109, 3359–3364. https://doi.org/10.1073/pnas.1115967109
Thi, M. M., Suadicani, S. O., Schaffler, M. B., Weinbaum, S., & Spray, D. C. (2013). Mechanosensory Responses of Osteocytes to Physiological Forces Occur along Processes and Not Cell Body and Require ΑVβ3 Integrin. Proceedings of the National Academy of Sciences of the United States of America, 110, 21012–21017. https://doi.org/10.1073/pnas.1321210110
Shackelford, L. C., LeBlanc, A. D., Driscoll, T. B., Evans, H. J., Rianon, N. J., Smith, S. M., Spector, E., Feeback, D. L., & Lai, D. (2004). Resistance Exercise as a countermeasure to Disuse-Induced Bone loss. Journal of Applied Physiology, 97, 119–129. https://doi.org/10.1152/japplphysiol.00741.2003
Orwoll, E. S., Adler, R. A., Amin, S., Binkley, N., Lewiecki, E. M., Petak, S. M., Shapses, S. A., Sinaki, M., Watts, N. B., & Sibonga, J. D. (2013). Skeletal health in long-duration astronauts: Nature, Assessment, and Management Recommendations from the NASA Bone Summit. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 28, 1243–1255. https://doi.org/10.1002/jbmr.1948
Uda, Y., Azab, E., Sun, N., Shi, C., & Pajevic, P. D. (2017). Osteocyte Mechanobiology. Current Osteoporosis Reports, 15, 318–325. https://doi.org/10.1007/s11914-017-0373-0
Davis, H. M., Pacheco-Costa, R., Atkinson, E. G., Brun, L. R., Gortazar, A. R., Harris, J., Hiasa, M., Bolarinwa, S. A., Yoneda, T., Ivan, M., et al. (2017). Disruption of the Cx43/MiR21 pathway leads to Osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell, 16, 551–563. https://doi.org/10.1111/acel.12586
Noble, B. S., Peet, N., Stevens, H. Y., Brabbs, A., Mosley, J. R., Reilly, G. C., Reeve, J., Skerry, T. M., & Lanyon, L. E. (2003). Mechanical loading: Biphasic osteocyte survival and targeting of osteoclasts for Bone Destruction in Rat cortical bone. American Journal Of Physiology. Cell Physiology, 284, C934–C943. https://doi.org/10.1152/ajpcell.00234.2002
Lloyd, S. A., Lang, C. H., Zhang, Y., Paul, E. M., Laufenberg, L. J., Lewis, G. S., & Donahue, H. J. (2014). Interdependence of muscle atrophy and bone loss Induced by Mechanical Unloading. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 29, 1118–1130. https://doi.org/10.1002/jbmr.2113
Werner, S. L., Sharma, R., Woodruff, K., Horn, D., Harris, S. E., Gorin, Y., Lee, D. Y., Hua, R., Gu, S., Fajardo, R. J., et al. (2020). CSF-1 in Osteocytes inhibits Nox4-Mediated oxidative stress and promotes normal bone homeostasis. JBMR Plus, 4, e10080. https://doi.org/10.1002/jbm4.10080
Tomkinson, A., Reeve, J., Shaw, R. W., & Noble, B. S. (1997). The death of Osteocytes via apoptosis accompanies estrogen withdrawal in human bone. Journal of Clinical Endocrinology and Metabolism, 82, 3128–3135. https://doi.org/10.1210/jcem.82.9.4200
Weinstein, R. S., Nicholas, R. W., & Manolagas, S. C. (2000). Apoptosis of Osteocytes in Glucocorticoid-Induced osteonecrosis of the hip. Journal of Clinical Endocrinology and Metabolism, 85, 2907–2912. https://doi.org/10.1210/jcem.85.8.6714
Plotkin, L. I., Mathov, I., Aguirre, J. I., Parfitt, A. M., Manolagas, S. C., & Bellido, T. (2005). Mechanical stimulation prevents Osteocyte apoptosis: Requirement of integrins, src kinases, and ERKs. American Journal Of Physiology. Cell Physiology, 289, C633–C643. https://doi.org/10.1152/ajpcell.00278.2004
Choi, J. U. A., Kijas, A. W., Lauko, J., & Rowan, A. E. (2021). The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Frontiers in Cell and Developmental Biology, 9, 770143. https://doi.org/10.3389/fcell.2021.770143
Yahara, Y., Nguyen, T., Ishikawa, K., Kamei, K., & Alman, B. A. (2022). The Origins and Roles of Osteoclasts in Bone Development, Homeostasis and Repair. Development, 149, dev199908. https://doi.org/10.1242/dev.199908
Al-Bari, A. A., & Al Mamun, A. (2020). Current advances in regulation of bone homeostasis. FASEB BioAdvances, 2, 668–679. https://doi.org/10.1096/fba.2020-00058
Yu, J., & Canalis, E. (2020). Notch and the regulation of Osteoclast differentiation and function. Bone, 138, 115474. https://doi.org/10.1016/j.bone.2020.115474
Sarrazin, S., & Sieweke, M. (2011). Integration of cytokine and transcription factor signals in hematopoietic stem cell commitment. Seminars in Immunology, 23, 326–334. https://doi.org/10.1016/j.smim.2011.08.011
Sarrazin, S., Mossadegh-Keller, N., Fukao, T., Aziz, A., Mourcin, F., Vanhille, L., Kelly Modis, L., Kastner, P., Chan, S., Duprez, E., et al. (2009). MafB restricts M-CSF-Dependent myeloid commitment divisions of hematopoietic stem cells. Cell, 138, 300–313. https://doi.org/10.1016/j.cell.2009.04.057
Mossadegh-Keller, N., Sarrazin, S., Kandalla, P. K., Espinosa, L., Stanley, E. R., Nutt, S. L., Moore, J., & Sieweke, M. H. (2013). M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature, 497, 239–243. https://doi.org/10.1038/nature12026
Kwon, O. H., Lee, C. K., Lee, Y. I., Paik, S. G., & Lee, H. J. (2005). The hematopoietic transcription factor PU.1 regulates RANK gene expression in myeloid progenitors. Biochemical and Biophysical Research Communications, 335, 437–446. https://doi.org/10.1016/j.bbrc.2005.07.092
Sucur, A., Jajic, Z., Artukovic, M., Matijasevic, M. I., Anic, B., Flegar, D., Markotic, A., Kelava, T., Ivcevic, S., Kovacic, N., et al. (2017). Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Research & Therapy, 19, 142. https://doi.org/10.1186/s13075-017-1337-6
Jung, Y., Wang, J., Schneider, A., Sun, Y. X., Koh-Paige, A. J., Osman, N. I., McCauley, L. K., & Taichman, R. S. (2006). Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for Stem Cell Homing. Bone, 38, 497–508. https://doi.org/10.1016/j.bone.2005.10.003
Yu, X., Huang, Y., Collin-Osdoby, P., & Osdoby, P. (2003). Stromal cell-derived Factor-1 (SDF-1) recruits osteoclast precursors by inducing Chemotaxis, Matrix Metalloproteinase-9 (MMP-9) activity, and collagen transmigration. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 18, 1404–1418. https://doi.org/10.1359/jbmr.2003.18.8.1404
Lee, D., Shin, K. J., Kim, D. W., Yoon, K. A., Choi, Y. J., Lee, B. N. R., & Cho, J. Y. (2018). CCL4 enhances Preosteoclast Migration and its receptor CCR5 downregulation by RANKL promotes osteoclastogenesis. Cell Death and Disease, 9, 495. https://doi.org/10.1038/s41419-018-0562-5
Koizumi, K., Saitoh, Y., Minami, T., Takeno, N., Tsuneyama, K., Miyahara, T., Nakayama, T., Sakurai, H., Takano, Y., Nishimura, M., et al. (2009). Role of CX3CL1/Fractalkine in Osteoclast differentiation and bone Resorption1. The Journal of Immunology, 183, 7825–7831. https://doi.org/10.4049/jimmunol.0803627
Maeda, K., Kobayashi, Y., Udagawa, N., Uehara, S., Ishihara, A., Mizoguchi, T., Kikuchi, Y., Takada, I., Kato, S., Kani, S., et al. (2012). Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances Osteoclastogenesis. Nature Medicine, 18, 405–412. https://doi.org/10.1038/nm.2653
Kim, J. M., Lin, C., Stavre, Z., Greenblatt, M. B., & Shim, J. H. (2020). Osteoblast-osteoclast communication and bone homeostasis. Cells, 9. https://doi.org/10.3390/cells9092073
Yao, Z., Xing, L., Qin, C., Schwarz, E. M., & Boyce, B. F. (2008). Osteoclast Precursor Interaction with Bone Matrix induces osteoclast formation directly by an Interleukin-1-Mediated autocrine mechanism. Journal of Biological Chemistry, 283, 9917–9924. https://doi.org/10.1074/jbc.M706415200
Udagawa, N., Takahashi, N., Jimi, E., Matsuzaki, K., Tsurukai, T., Itoh, K., Nakagawa, N., Yasuda, H., Goto, M., Tsuda, E., et al. (1999). Osteoblasts/Stromal cells stimulate osteoclast activation through expression of Osteoclast differentiation Factor/RANKL but not macrophage colony-stimulating factor: Receptor activator of NF-Kappa B ligand. Bone, 25, 517–523. https://doi.org/10.1016/s8756-3282(99)00210-0
Nakashima, T., Kobayashi, Y., Yamasaki, S., Kawakami, A., Eguchi, K., Sasaki, H., & Sakai, H. (2000). Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-KappaB ligand: Modulation of the expression by osteotropic factors and cytokines. Biochemical and Biophysical Research Communications, 275, 768–775. https://doi.org/10.1006/bbrc.2000.3379
Kaneshita, Y., Goda, S., & Kawamoto, T. (2007). The Effect of Matrix Metalloproteinase-9 on the differentiation into Osteoclast cells on RAW264 cells. Orthod Waves, 66, 122–128. https://doi.org/10.1016/j.odw.2007.09.003
Xiong, J., Onal, M., Jilka, R. L., Weinstein, R. S., Manolagas, S. C., & O’Brien, C. A. (2011). Matrix-embedded cells control osteoclast formation. Nature Medicine, 17, 1235–1241. https://doi.org/10.1038/nm.2448
Ru, J., Wang, Y., & Osteocyte Apoptosis. (2020). The Roles and Key Molecular Mechanisms in Resorption-Related Bone Diseases. Cell Death and Disease, 11, 846. https://doi.org/10.1038/s41419-020-03059-8
Feng, W., Guo, J., & Li, M. (2019). RANKL-Independent modulation of Osteoclastogenesis. Journal of Oral Biosciences, 61, 16–21. https://doi.org/10.1016/j.job.2019.01.001
Wu, Q., Zhou, X., Huang, D., JI, Y., & Kang, F. (2017). IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in Vitro Cellular Physiology and Biochemistry, 41, 1360–1369. https://doi.org/10.1159/000465455
Gangenahalli, G. U., Gupta, P., Saluja, D., Verma, Y. K., Kishore, V., Chandra, R., Sharma, R. K., & Ravindranath, T. (2005). Stem cell fate specification: Role of Master Regulatory switch transcription factor PU.1 in Differential hematopoiesis. Stem Cells and Development, 14, 140–152. https://doi.org/10.1089/scd.2005.14.140
Iwasaki, H., Somoza, C., Shigematsu, H., Duprez, E. A., Iwasaki-Arai, J., Mizuno, S., Arinobu, Y., Geary, K., Zhang, P., Dayaram, T., et al. (2005). Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood, 106, 1590–1600. https://doi.org/10.1182/blood-2005-03-0860
Zhang, D. E., Hetherington, C. J., Chen, H. M., & Tenen, D. G. (1994). The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Molecular and Cellular Biology, 14, 373–381. https://doi.org/10.1128/mcb.14.1.373-381.1994
Carey, H., Hildreth, B., Geisler, J., Nickel, M., Cabrera, J., Ghosh, S., Jiang, Y., Yan, J., Lee, J., Makam, S., et al. (2018). Enhancer variants reveal a conserved transcription factor network governed by PU.1 during osteoclast differentiation. Bone Research, 6. https://doi.org/10.1038/s41413-018-0011-1
Sharma, S. M., Bronisz, A., Hu, R., Patel, K., Mansky, K. C., Sif, S., & Ostrowski, M. C. (2007). MITF and PU.1 Recruit P38 MAPK and NFATc1 to Target genes during Osteoclast Differentiation*. Journal of Biological Chemistry, 282, 15921–15929. https://doi.org/10.1074/jbc.M609723200
Søe, K. (2020). Osteoclast Fusion: Physiological regulation of multinucleation through heterogeneity—potential implications for drug sensitivity. International Journal of Molecular Sciences, 21. https://doi.org/10.3390/ijms21207717
Miyamoto, T., Arai, F., Ohneda, O., Takagi, K., Anderson, D. M., & Suda, T. (2000). An adherent Condition is required for formation of multinuclear osteoclasts in the Presence of Macrophage colony-stimulating factor and receptor activator of Nuclear factor ΚB ligand. Blood, 96, 4335–4343. https://doi.org/10.1182/blood.V96.13.4335
Grzesik, W. J., Robey, P. G., Bone Matrix, R. G. D., & Glycoproteins. (1994). Immunolocalization and Interaction with Human Primary Osteoblastic Bone cells in Vitro. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 9, 487–496. https://doi.org/10.1002/jbmr.5650090408
Barrow, A. D., Raynal, N., Andersen, T. L., Slatter, D. A., Bihan, D., Pugh, N., Cella, M., Kim, T., Rho, J., Negishi-Koga, T., et al. (2011). OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-Deficient humans and mice. Journal of Clinical Investigation, 121, 3505–3516. https://doi.org/10.1172/JCI45913
Nedeva, I. R., Vitale, M., Elson, A., Hoyland, J. A., & Bella, J. (2021). Role of OSCAR signaling in osteoclastogenesis and bone disease. Frontiers in Cell and Developmental Biology, 9, 641162.https://doi.org/10.3389/fcell.2021.641162
Søe, K., Delaisse, J. M., & Borggaard, X. G. (2021). Osteoclast formation at the bone Marrow/Bone surface interface: Importance of structural elements, Matrix, and Intercellular Communication. Seminars in Cell & Developmental Biology, 112, 8–15. https://doi.org/10.1016/j.semcdb.2020.05.016
Yagi, M., Ninomiya, K., Fujita, N., Suzuki, T., Iwasaki, R., Morita, K., Hosogane, N., Matsuo, K., Toyama, Y., Suda, T., et al. (2007). Induction of DC-STAMP by Alternative activation and downstream Signaling Mechanisms. Journal of Bone and Mineral Research, 22, 992–1001. https://doi.org/10.1359/jbmr.070401
Blangy, A., Bompard, G., Guerit, D., Marie, P., Maurin, J., Morel, A., & Vives, V. (2020). The Osteoclast Cytoskeleton – Current understanding and therapeutic perspectives for osteoporosis. Journal of Cell Science, 133, jcs244798. https://doi.org/10.1242/jcs.244798
Paiva, K. B. S., & Granjeiro, J. M. (2017). Chapter Six - Matrix Metalloproteinases in Bone Researchorption, Remodeling, and Repair. In Khalil, R. A. B. T.-P. in M. B. & T.S (Eds.) Matrix Metalloproteinases and Tissue Remodeling in Health and Disease: Target Tissues and Therapy (Vol. 148, pp. 203–303). Academic. ISBN 1877-1173.
Sundaram, K., Nishimura, R., Senn, J., Youssef, R. F., London, S. D., & Reddy, S. (2007). V RANK ligand signaling modulates the Matrix Metalloproteinase-9 gene expression during osteoclast differentiation. Experimental Cell Research, 313, 168–178. https://doi.org/10.1016/j.yexcr.2006.10.001
Dai, R., Wu, Z., Chu, H. Y., Lu, J., Lyu, A., Liu, J., Zhang, G., & Cathepsin, K. (2020). The action in and beyond bone. Frontiers in Cell and Developmental Biology, 8, https://doi.org/10.3389/fcell.2020.00433
Roscher, A., Hasegawa, T., Dohnke, S., Ocaña-Morgner, C., Amizuka, N., Jessberger, R., & Garbe, A. I. (2016). The F-Actin modulator SWAP-70 Controls Podosome patterning in osteoclasts. Bone Reports, 5, 214–221. https://doi.org/10.1016/j.bonr.2016.07.002
Dou, C., Cao, Z., Yang, B., Ding, N., Hou, T., Luo, F., Kang, F., Li, J., Yang, X., Jiang, H., et al. (2016). Changing expression profiles of LncRNAs, MRNAs, CircRNAs and MiRNAs during Osteoclastogenesis. Scientific Reports, 6, 21499. https://doi.org/10.1038/srep21499
Wu, H., Xu, G., & Li, Y. P. (2009). Atp6v0d2 is an essential component of the osteoclast-specific Proton Pump that mediates extracellular acidification in bone resorption. Journal of Bone and Mineral Research: Official Journal of the American Society for Bone and Mineral Research, 24, 871–885. https://doi.org/10.1359/jbmr.081239
Kim, B. H., Oh, J. H., & Lee, N. K. (2017). The inactivation of ERK1/2, P38 and NF-KB is involved in the down-regulation of osteoclastogenesis and function by A2B adenosine receptor stimulation. Molecules and Cells, 40, 752–760. https://doi.org/10.14348/molcells.2017.0098
Liu, Y., Shi, Z., Silveira, A., Liu, J., Sawadogo, M., Yang, H., & Feng, X. (2003). Involvement of Upstream Stimulatory factors 1 and 2 in RANKL-Induced transcription of tartrate-resistant acid phosphatase gene during osteoclast differentiation. Journal of Biological Chemistry, 278, 20603–20611. https://doi.org/10.1074/jbc.M212093200
Zhu, L., Tang, Y., Li, X. Y., Keller, E. T., Yang, J., Cho, J. S., Feinberg, T. Y., & Weiss, S. J. (2020). Osteoclast-mediated bone resorption is controlled by a Compensatory Network of secreted and membrane-tethered metalloproteinases. Science Translational Medicine, 12. https://doi.org/10.1126/scitranslmed.aaw6143
Blair, H., Yaroslavskiy, B., Robinson, L., Mapara, M., Pangrazio, A., Guo, L., Chen, K., Vezzoni, P., Tolar, J., & Orchard, P. (2009). Osteopetrosis with Micro-Lacunar Resorption due to defective integrin Organization. Laboratory Investigation, 89, 1007–1017. https://doi.org/10.1038/labinvest.2009.58
Drake, F. H., Dodds, R. A., James, I. E., Connor, J. R., Debouck, C., Richardson, S., Lee-Rykaczewski, E., Coleman, L., Rieman, D., Barthlow, R., et al., Cathepsin, K., but, Cathepsins, N. (1996). B, L, or S, is abundantly expressed in human osteoclasts (∗). Journal of Biological Chemistry, 271, 12511–12516. https://doi.org/10.1074/jbc.271.21.12511
Hayman, A. (2008). Tartrate-resistant acid phosphatase (TRAP) and the Osteoclast/Immune Cell Dichotomy. Autoimmunity, 41, 218–223. https://doi.org/10.1080/08916930701694667
Paschos, N. K., Brown, W. E., Eswaramoorthy, R., Hu, J. C., & Athanasiou, K. A. (2015). Advances in tissue Engineering through Stem Cell-Based co-culture. Journal of Tissue Engineering and Regenerative Medicine, 9, 488–503. https://doi.org/10.1002/term.1870
Singh, V. K., Kalsan, M., Kumar, N., Saini, A., & Chandra, R. (2015). Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in Cell and Developmental Biology, 3(2). https://doi.org/10.3389/fcell.2015.00002
Okita, K., Ichisaka, T., & Yamanaka, S. (2007). Generation of Germline-Competent Induced Pluripotent Stem cells. Nature, 448, 313–317. https://doi.org/10.1038/nature05934
Jeon, O. H., Panicker, L. M., Lu, Q., Chae, J. J., Feldman, R. A., & Elisseeff, J. H. (2016). Human IPSC-Derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Scientific Reports, 6, 26761. https://doi.org/10.1038/srep26761
Zhang, J., Chen, M., Liao, J., Chang, C., Liu, Y., Padhiar, A. A., Zhou, Y., & Zhou, G. (2021). Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Hold Lower Heterogeneity and Great Promise in Biological Research and Clinical Applications. Frontiers in Cell and Developmental Biology, 9, 716907. https://doi.org/10.3389/fcell.2021.716907
Gultian, K. A., Gandhi, R., Sarin, K., Sladkova-Faure, M., Zimmer, M., de Peppo, G. M., & Vega, S. L. (2022). Human Induced mesenchymal stem cells display increased sensitivity to Matrix Stiffness. Scientific Reports, 12, 8483. https://doi.org/10.1038/s41598-022-12143-2
Dupuis, V., & Oltra, E. (2021). Methods to produce Induced pluripotent stem cell-derived mesenchymal stem cells: Mesenchymal stem cells from Induced Pluripotent Stem cells. World Journal of Stem Cells, 13, 1094–1111. https://doi.org/10.4252/wjsc.v13.i8.1094
Phillips, M. D., Kuznetsov, S. A., Cherman, N., Park, K., Chen, K. G., McClendon, B. N., Hamilton, R. S., McKay, R. D. G., Chenoweth, J. G., Mallon, B. S., et al. (2014). Directed differentiation of Human Induced Pluripotent Stem cells toward bone and cartilage: In Vitro versus in vivo assays. Stem Cells Translational Medicine, 3, 867–878. https://doi.org/10.5966/sctm.2013-0154
Zou, L., Luo, Y., Chen, M., Wang, G., Ding, M., Petersen, C. C., Kang, R., Dagnaes-Hansen, F., Zeng, Y., Lv, N., et al. (2013). A simple method for deriving functional MSCs and Applied for Osteogenesis in 3D scaffolds. Scientific Reports, 3. https://doi.org/10.1038/srep02243
Zhu, H., Kimura, T., Swami, S., & Wu, J. Y. (2019). Pluripotent stem cells as a source of osteoblasts for bone tissue regeneration. Biomaterials, 196, 31–45. https://doi.org/10.1016/j.biomaterials.2018.02.009
Hu, J., Smith Callahan, L., Feng, K., Liu, X., Sun, H., & Ma, P. (2010). Response of human embryonic stem Cell???Derived mesenchymal stem cells to osteogenic factors and architectures of materials during in Vitro Osteogenesis. Tissue Engineering Part A, 16, 3507–3514. https://doi.org/10.1089/ten.TEA.2010.0097
Kurosawa, H. (2007). Methods for inducing embryoid body formation: In Vitro differentiation system of embryonic stem cells. Journal of Bioscience and Bioengineering, 103, 389–398. https://doi.org/10.1263/jbb.103.389
Mora-Roldan, G. A., Ramirez-Ramirez, D., Pelayo, R., & Gazarian, K. (2021). Assessment of the hematopoietic differentiation potential of human pluripotent stem cells in 2D and 3D Culture Systems. Cells, 10. https://doi.org/10.3390/cells10112858
Lim, W. F., Inoue-Yokoo, T., Tan, K. S., Lai, M. I., & Sugiyama, D. (2013). Hematopoietic cell differentiation from Embryonic and Induced Pluripotent Stem cells. Stem Cell Research & Therapy, 4. https://doi.org/10.1186/scrt222
Salvagiotto, G., Burton, S., Daigh, C. A., Rajesh, D., Slukvin, I. I., Seay, N. J. A., & Defined. (2011). Feeder-Free, serum-free system to Generate in Vitro hematopoietic progenitors and differentiated blood cells from HESCs and HiPSCs. PLoS One, 6, e17829.
Rössler, U., Hennig, A. F., Stelzer, N., Bose, S., Kopp, J., Søe, K., Cyganek, L., Zifarelli, G., Ali, S., von der Hagen, M., et al. (2021). Efficient generation of osteoclasts from Human Induced Pluripotent Stem cells and functional investigations of Lethal CLCN7-Related osteopetrosis. Journal of Bone and Mineral Research, 36, 1621–1635. https://doi.org/10.1002/jbmr.4322
Khademhosseini, A., Ashammakhi, N., Karp, J. M., Gerecht, S., Ferreira, L., Annabi, N., Darabi, M. A., Sirabella, D., Vunjak-Novakovic, G., & Langer, R. (2020). In Lanza, R., Langer, R., Vacanti, J. P., Atala, A.B.T.-P. of T.E. Chapter 27 - Embryonic Stem Cells as a Cell Source for Tissue Engineering (Fifth E., Eds., pp.467–490). Academic. ISBN 978-0-12-818422-6.
Sobacchi, C., Palagano, E., Villa, A., & Menale, C. (2017). Soluble factors on stage to direct mesenchymal stem cells fate. Frontiers in Bioengineering and Biotechnology, 5, 32. https://doi.org/10.3389/fbioe.2017.00032
Lobov, A., Malashicheva, A., & Osteogenic Differentiation. (2022). A Universal Cell Program of heterogeneous mesenchymal cells or a similar Extracellular Matrix mineralizing phenotype? Biological Communications, 67, 32–48. https://doi.org/10.21638/spbu03.2022.104
Langenbach, F., & Handschel, J. (2013). Effects of Dexamethasone, ascorbic acid and β-Glycerophosphate on the osteogenic differentiation of stem cells in Vitro. Stem Cell Research & Therapy, 4, 117. https://doi.org/10.1186/scrt328
Zujur, D., Kanke, K., Onodera, S., Tani, S., Lai, J., Azuma, T., Xin, X., Lichtler, A. C., Rowe, D. W., Saito, T., et al. (2020). Stepwise strategy for Generating osteoblasts from human pluripotent stem cells under fully defined Xeno-Free Conditions with small-molecule inducers. Regenerative Therapy, 14, 19–31. https://doi.org/10.1016/j.reth.2019.12.010
Kawai, S., Yoshitomi, H., Sunaga, J., Alev, C., Nagata, S., Nishio, M., Hada, M., Koyama, Y., Uemura, M., Sekiguchi, K., et al. (2019). In Vitro Bone-like nodules generated from patient-derived IPSCs recapitulate pathological bone phenotypes. Nature Biomedical Engineering, 3, 1–13. https://doi.org/10.1038/s41551-019-0410-7
Kang, H., Shih, Y. R. V., Nakasaki, M., Kabra, H., & Varghese, S. (2023). Small Molecule–Driven Direct Conversion of Human pluripotent stem cells into functional osteoblasts. Science Advances, 2, e1600691. https://doi.org/10.1126/sciadv.1600691
Hong, H., Shi, Z., Qiao, P., Li, H., McCoy, E. M., Mao, P., Xu, H., Feng, X., & Wang, S. (2013). Interleukin-3 plays dual roles in Osteoclastogenesis by promoting the development of osteoclast progenitors but inhibiting the osteoclastogenic process. Biochemical and Biophysical Research Communications, 440, 545–550. https://doi.org/10.1016/j.bbrc.2013.09.098
De Witte, T. M., Fratila-Apachitei, L. E., Zadpoor, A. A., & Peppas, N. A. (2018). Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regenerative Biomaterials, 5, 197–211. https://doi.org/10.1093/rb/rby013
De Pieri, A., Rochev, Y., & Zeugolis, D. I. (2021). Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. NPJ Regenerative Medicine, 6. https://doi.org/10.1038/s41536-021-00133-3
Haraguchi, Y., Shimizu, T., Sasagawa, T., Sekine, H., Sakaguchi, K., Kikuchi, T., Sekine, W., Sekiya, S., Yamato, M., Umezu, M., et al. (2012). Fabrication of Functional three-dimensional tissues by stacking cell sheets in Vitro. Nature Protocols, 7, 850–858. https://doi.org/10.1038/nprot.2012.027
Okawa, H., Kayashima, H., Sasaki, J. I., Miura, J., Kamano, Y., Kosaka, Y., Imazato, S., Yatani, H., Matsumoto, T., & Egusa, H. (2016). Scaffold-free fabrication of osteoinductive cellular constructs using mouse gingiva-derived induced pluripotent stem cells. Stem Cells International, 2016, 6240794. https://doi.org/10.1155/2016/6240794
Ozasa, R., Matsugaki, A., Isobe, Y., Saku, T., Yun, H. S., & Nakano, T. (2018). Construction of Human Induced Pluripotent Stem Cell-Derived oriented bone matrix microstructure by using in Vitro Engineered Anisotropic Culture Model. Journal of Biomedical Materials Research Part A, 106, 360–369. https://doi.org/10.1002/jbm.a.36238
Kang, H., Shih, Y. R. V., Hwang, Y., Wen, C., Rao, V., Seo, T., & Varghese, S. (2014). Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of Human Induced Pluripotent Stem cells. Acta Biomaterialia, 10, 4961–4970. https://doi.org/10.1016/j.actbio.2014.08.010
Mazzoni, E., Mazziotta, C., Iaquinta, M. R., Lanzillotti, C., Fortini, F., D’Agostino, A., Trevisiol, L., Nocini, R., Barbanti-Brodano, G., Mescola, A., et al. (2020). Enhanced osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by a Hybrid Hydroxylapatite/Collagen Scaffold. Frontiers in Cell and Developmental Biology, 8, 610570. https://doi.org/10.3389/fcell.2020.610570
Zhang, Y., Xia, L., Zhai, D., Shi, M., Luo, Y., Feng, C., Fang, B., Yin, J., Chang, J., & Wu, C. (2015). Mesoporous bioactive glass nanolayer-functionalized 3D-printed scaffolds for accelerating osteogenesis and angiogenesis. Nanoscale, 7, 19207–19221. https://doi.org/10.1039/c5nr05421d
Tariverdian, T., Sefat, F., Gelinsky, M., & Mozafari, M. (2019). 10 - Scaffold for bone tissue engineering. In Mozafari, M., Sefat, F., & Atala, A. (Eds.) Handbook of Tissue Engineering Scaffolds: Volume One (pp. 189–209). Woodhead Publishing Series in Biomaterials; Woodhead Publishing. ISBN 978-0-08-102563-5.
Amirazad, H., Dadashpour, M., & Zarghami, N. (2022). Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. Journal of Biological Engineering, 16, 1. https://doi.org/10.1186/s13036-021-00282-5
Borciani, G., Montalbano, G., Baldini, N., Cerqueni, G., Vitale-Brovarone, C., & Ciapetti, G. (2020). Co–culture systems of osteoblasts and osteoclasts: simulating in vitro bone remodeling in regenerative approaches. Acta Biomaterialia, 108, 22–45. https://doi.org/10.1016/j.actbio.2020.03.043
Zheng, Z., Patel, M., & Patel, R. (2022). Hyaluronic acid-based materials for bone regeneration: A review. Reactive & Functional Polymers, 171, 105151. https://doi.org/10.1016/j.reactfunctpolym.2021.105151
Almeida, A. R., Bessa-Gonçalves, M., Vasconcelos, D. M., Barbosa, M. A., & Santos, S. G. (2020). Osteoclasts degrade fibrinogen scaffolds and induce mesenchymal Stem/Stromal osteogenic differentiation. Journal of Biomedical Materials Research. Part A, 108, 851–862. https://doi.org/10.1002/jbm.a.36863
Bernhardt, A., Skottke, J., von Witzleben, M., & Gelinsky, M. (2021). Triple culture of primary human osteoblasts, osteoclasts and osteocytes as an in vitro bone model. International Journal of Molecular Sciences, 22. https://doi.org/10.3390/ijms22147316
Mansoorifar, A., Gordon, R., Bergan, R., & Bertassoni, L. E. (2021). Bone-on-a-Chip: Microfluidic technologies and microphysiologic models of bone tissue. Advanced Functional Materials, 31. https://doi.org/10.1002/adfm.202006796
Yang, X., Tang, Q., Lai, C., Wu, K., & Shi, X. (2021). Applications and prospects of microfluidic chips in orthopaedic diseases. Frontiers of Materials, 7, 610558. https://doi.org/10.3389/fmats.2020.610558
Mejía-Salazar, J. R., Rodrigues Cruz, K., Materón Vásques, E. M., & Novais de Oliveira, O. J. (2020). Microfluidic point-of-care devices: new trends and future prospects for EHealth Diagnostics. Sensors (Basel), 20. https://doi.org/10.3390/s20071951
Truesdell, S. L., George, E. L., Van Vranken, C. C., Saunders, M. M. A., & Lab-On. (2020). -A-Chip platform for stimulating osteocyte mechanotransduction and analyzing functional outcomes of bone remodeling. Journal of Visualized Experiments: Jove. https://doi.org/10.3791/61076
Park, Y., Cheong, E., Kwak, J. G., Carpenter, R., Shim, J. H., & Lee, J. (2021). Trabecular bone Organoid Model for studying the regulation of localized bone remodeling. Science Advances, 7. https://doi.org/10.1126/sciadv.abd6495
Fatehullah, A., Tan, S. H., & Barker, N. (2016). Organoids as an in Vitro Model of Human Development and Disease. Nature Cell Biology, 18, 246–254. https://doi.org/10.1038/ncb3312
Chen, S., Chen, X., Geng, Z., & Su, J. (2022). The Horizon of Bone Organoid: A perspective on construction and application. Bioactive Materials, 18, 15–25. https://doi.org/10.1016/j.bioactmat.2022.01.048
Iordachescu, A., Hughes, E. A. B., Joseph, S., Hill, E. J., Grover, L. M., & Metcalfe, A. D. (2021). Trabecular bone organoids: A Micron-Scale ‘Humanised’ Prototype designed to study the Effects of Microgravity and Degeneration. NPJ Microgravity, 7, 17. https://doi.org/10.1038/s41526-021-00146-8
Grosso, A., Burger, M. G., Lunger, A., Schaefer, D. J., Banfi, A., & Di Maggio, N. (2017). It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Frontiers in Bioengineering and Biotechnology, 5, 68. https://doi.org/10.3389/fbioe.2017.00068
Bessy, T., Itkin, T., & Passaro, D. (2021). Bioengineering the bone marrow vascular niche. Frontiers in Cell and Developmental Biology, 9, 645496. https://doi.org/10.3389/fcell.2021.645496
Funding
This work was supported by Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2021-1075 dated 09/28/2021).
Author information
Authors and Affiliations
Contributions
OK - wrote a manuscript, provided graphics and comments.
IN - conceived idea, provided critical revisions of the manuscript. Both authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
It is a review paper.
Consent to Participate
It is a review paper. No animals or humans have been involved.
Consent to Publish
All authors gave consent to publish.
Competing Interests
Authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krasnova, O., Neganova, I. Assembling the Puzzle Pieces. Insights for in Vitro Bone Remodeling. Stem Cell Rev and Rep 19, 1635–1658 (2023). https://doi.org/10.1007/s12015-023-10558-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10558-6