Abstract
Purpose:
This work compiles the characteristics of bone cells involved in the physiological bone remodeling.
Methods:
A narrative review of the literature was performed.
Results:
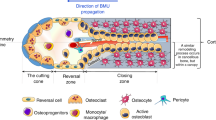
Remodeling is a different process from modeling. Remodeling allows old or damaged bone tissue to be renewed, ensuring the maintenance of bone fracture resistance, as well as maintaining calcium and phosphorus homeostasis. We present the role of osteoclasts, a multinucleated cell with hematopoietic origin responsible for resorbing bone. The formation of osteoclasts depends on the cytokines macrophage colony stimulating factor (M-CSF) and receptor activator of NF-kB ligand (RANKL) and can be blocked by osteoprotegerin. Furthermore, this review highlights the features of osteoblasts, polarized cubic cells of mesenchymal origin that deposit bone and also covers osteocytes and bone lining cells. This review presents the five fundamental phases of bone remodeling and addresses aspects of its regulation through hormones and growth factors.
Conclusions:
Knowledge of the current concepts of physiological bone remodeling is necessary for the study of the different pathologies that affect the bone tissue and thus helps in the search for new therapies.

Similar content being viewed by others
References
Kodama J, Kaito T (2020) Osteoclast multinucleation: review of current literature. Int J Mol Sci 21(16):1–35. https://doi.org/10.3390/ijms21165685
Guo YF, Su T, Yang M, Li C, jun, Guo Q, Xiao Y, Huang Y, Liu Y, Luo X (2021) The role of autophagy in bone homeostasis. J Cell Physiol 236(6):4152–4173. https://doi.org/10.1002/jcp.30111
Pant A, Paul E, Niebur GL, Vahdati A (2021) Integration of mechanics and biology in computer simulation of bone remodeling. Prog Biophys Mol Biol 164:33–45. https://doi.org/10.1016/j.pbiomolbio.2021.05.001
Sims NA, Martin TJ (2020) Osteoclasts provide coupling signals to osteoblast lineage cells through multiple mechanisms. Annu Rev Physiol 82:507–529. https://doi.org/10.1146/annurev-physiol-021119-034425
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C (2018) Osteoblast-osteoclast interactions. Connect Tissue Res 59(2):99–107. https://doi.org/10.1080/03008207.2017.1290085
Singh S, Bray TJP, Hall-Craggs MA (2018) Quantifying bone structure, micro-architecture, and pathophysiology with MRI. Clin Radiol 73(3):221–230. https://doi.org/10.1016/j.crad.2017.12.010
Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C, Tsuda E (2021) Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 2021;39(1):19–26. https://doi.org/10.1007/s00774-020-01162-6
Nagy V, Penninger JM (2015) The RANKL-RANK story. Gerontology 61(6):534–542. https://doi.org/10.1159/000371845
Bonewald LF (2017) The role of the Osteocyte in Bone and Nonbone Disease. Endocrinol Metab Clin North Am 46(1):1–18. https://doi.org/10.1016/j.ecl.2016.09.003
Buck DW, Dumanian GA (2012) Bone biology and physiology: Part I. the fundamentals. Plast Reconstr Surg 129(6):1314–1320. DOI: https://doi.org/10.1097/PRS.0b013e31824eca94
Boyce BF, Li J, Xing L, Yao Z (2018) Bone remodeling and the role of TRAF3 in Osteoclastic Bone Resorption. Front Immunol 9:2263. https://doi.org/10.3389/fimmu.2018.02263
Drissi H, Sanjay A (2016) The multifaceted osteoclast; Far and Beyond Bone Resorption. J Cell Biochem 1756:1753–1756. https://doi.org/10.1002/jcb.25560
Rossi M, Battafarano G, Pepe J, Minisola S, Fattore AD (2019) The endocrine function of osteocalcin regulated by bone resorption: a lesson from reduced and increased bone Mass Diseases. Int J Mol Sci 20(18):4502. https://doi.org/10.3390/ijms20184502
Amarasekara DS, Yun H, Kim S, Lee N, Kim H, Rho J (2018) Regulation of osteoclast differentiation by cytokine networks. Immune Netw 18(1):1–18. https://doi.org/10.4110/in.2018.18.e8
Anesi A, Generali L, Sandoni L, Pozzi S, Grande A (2019) From osteoclast differentiation to osteonecrosis of the jaw: Molecular and clinical insights. Int J Mol Sci 20(19):1–17. https://doi.org/10.3390/ijms20194925
Matsumoto T, Endo I (2021) RANKL as a target for the treatment of osteoporosis. J Bone Miner Metab 39(1):91–105. https://doi.org/10.1007/s00774-020-01153-7
Miyamoto T (2011) Regulators of osteoclast differentiation and cell-cell fusion. Keio J Med 60(4):101–105. https://doi.org/10.2302/kjm.60.101
Ralston SH (2017) Bone structure and metabolism. Med (United Kingdom) 45(9):560–564. https://doi.org/10.1016/j.mpmed.2017.06.008
Wang H, Zheng X, Zhang Y, Huang J, Zhou W, Li X, Tian H, Wang B, Xing D, Fu W, Chen T, Wang X, Zhang X, Wu A (2021) The endocrine role of bone: novel functions of bone-derived cytokines. Biochem Pharmacol 183. https://doi.org/10.1016/j.bcp.2020.114308
Salhotra A, Shah HN, Levi B, Longaker MT (2020) Mechanisms of bone development and repair. Nat Rev Mol Cell Biol 21:696–711. https://doi.org/10.1038/s41580-020-00279-w
Florencio-Silva R, Sasso GRDS, Sasso-Cerri E, Simões MJ, Cerri OS (2015) Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed Res Int. 2015. https://doi.org/10.1155/2015/421746
Mizoguchi T, Ono N (2021) The diverse origin of bone-forming osteoblasts. J Bone Miner Res 36(8):1432–1447. https://doi.org/10.1002/jbmr.4410
Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH (2020) Osteoblast-osteoclast communication and bone homeostasis. Cells 9(9):1–14. https://doi.org/10.3390/cells9092073
Grabowski P (2015) Physiology of bone. Endocr Dev 28:33–55. https://doi.org/10.1159/000380991
Duan P, Bonewald LF (2016) The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol 77:23–29. https://doi.org/10.1016/j.biocel.2016.05.015
Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, Kayma T, Saito M, Marumo K (2019) The regulation of bone metabolism and disordersby wnt signaling. Int J Mol Sci 20(22). https://doi.org/10.3390/ijms20225525
Donsante S, Palmisano B, Serafini M, Robey PG, Corsi A, Riminucci M (2021) From stem cells to bone-forming cells 1–23. https://doi.org/10.3390/ijms22083989
Haridy Y, Osenberg M, Hilger A, Manke I, Davesne D, Witzmann F (2021) Bone metabolism and evolutionary origin of osteocytes: novel application of FIB-SEM tomography. Sci Adv 7(14):1–12. https://doi.org/10.1126/sciadv.abb9113
Schaffler MB, Cheung WY, Majeska R, Kennedy O (2014) Osteocytes: Master orchestrators of bone. Calcif Tissue Int 94(1):5–24. https://doi.org/10.1007/s00223-013-9790-y
Ono T, Nakashima T (2022) Oral bone biology. J Oral Biosci 64(1):8–17. https://doi.org/10.1016/j.job.2022.01.008
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285(33):25103–25108. https://doi.org/10.1074/jbc.R109.041087
Manzini BM, Machado LMR, Noritomi PY, da Silva JVL (2021) Advances in Bone tissue engineering: A fundamental review. J Biosci 46(1) PMID: 33737501
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3Suppl3:131–139. https://doi.org/10.2215/CJN.04151206
Charles JF, Aliprantis AO (2014) Osteoclasts: more than ‘bone eaters’. Trends Mol Med 20(8):449–459. https://doi.org/10.1016/j.molmed.2014.06.001
Lerner UH, Kindstedt E, Lundberg P (2019) The critical interplay between bone resorbing and bone forming cells. J Clin Periodontol 46(S21):33–51. https://doi.org/10.1111/jcpe.13051
Kenkre JS, Bassett JHD (2018) The bone remodelling cycle. Ann Clin Biochem 55:308–327. https://doi.org/10.1177/0004563218759371
Katsimbri P (2017) The biology of normal bone remodelling. Eur J Cancer Care (Engl) 26(6):1–5. https://doi.org/10.1111/ecc.12740
Siddiqui JA, Partridge NC (2016) Physiological bone remodeling: systemic regulation and growth factor. Involv Physiol 31(3):233–245. https://doi.org/10.1152/physiol.00061.2014
Li C, Shi C, Kim J, Chen Y, Ni S, Jiang L, Zheng C, Li D, Hou J, Taichman RS, Sun H (2015) Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J Dent Res 94(3):455–463. https://doi.org/10.1177/0022034514566431
Kaur M, Nagpal M, Singh M (2020) Osteoblast-n-Osteoclast: making headway to osteoporosis treatment. Curr Drug Targets 21(16):1640–1651. https://doi.org/10.2174/1389450121666200731173522
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23(11):576–581. https://doi.org/10.1016/j.tem.2012.03.008
Silva BC, Bilezikian JP (2015) Chap. 15 - Anabolic and Catabolic Pathways of Parathyroid Hormone on the Skeleton, Editor(s): John P. Bilezikian,The Parathyroids (Third Edition), Academic Press: 233–244, ISBN 9780123971661 https://doi.org/10.1016/B978-0-12-397166-1.00015-1
Wein MN, Kronenberg HM (2018) Regulation of bone remodeling by parathyroid hormone. Cold Spring Harb Perspect Med 8(8). https://doi.org/10.1101/cshperspect.a031237
Raisz LG (1999) Prostaglandins and bone: physiology and pathophysiology. Osteoarthr Cartil 7(4):419–421. https://doi.org/10.1053/joca.1998.0230
Itkin T, Kaufmann KB, Gur-Cohen S, Ludin A, Lapidot T (2013) Fibroblast growth factor signaling promotes physiological bone remodeling and stem cell self-renewal. Curr Opin Hematol 20(3):237–244. https://doi.org/10.1097/MOH.0b013e3283606162
Crane JL, Cao X (2014) Function of matrix IGF-1 in coupling bone resorption and formation. J Mol Med (Berl) 92(2):107–115. https://doi.org/10.1007/s00109-013-1084-3
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no confict of interest to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Research involving human and animal participants
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maciel, G.B.M., Maciel, R.M. & Danesi, C.C. Bone cells and their role in physiological remodeling. Mol Biol Rep 50, 2857–2863 (2023). https://doi.org/10.1007/s11033-022-08190-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08190-7