Abstract
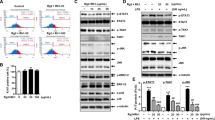
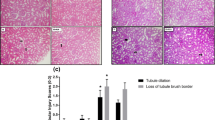
This study was conducted to compare the efficacy of the mouse hepatic and renal antioxidant systems against inflammation-induced oxidative stress. Increased Il-1 and Il-6 expressions, markers of inflammation, were represented by inflammation models in mouse liver and kidney tissues injected intraperitoneally with LPS. After establishing the model, the GSH level and the GSH/GSSG ratio, which are oxidative stress markers, were investigated in both tissues treated with LPS and the control group. The expression of Trx1, TrxR, and Txnip genes increased in the liver tissues of LPS-treated mice. In the kidney tissue, while Trx1 expression decreased, no change was observed in TrxR1 expression, and Txnip expression increased. In the kidneys, TRXR1 and GR activities decreased, whereas GPx activity increased. In both tissues, the TRXR1 protein expression decreased significantly, while TXNIP expression increased. In conclusion, different behaviors of antioxidant system members were observed during acute inflammation in both tissues. Additionally, it can be said that the kidney tissue is more sensitive and takes earlier measures than the liver tissue against cellular damage caused by inflammation and inflammation-induced oxidative stress.





Similar content being viewed by others
Data availability
The data created and analyzed during this study were added to the additional information files.
References
Wynn, T. A., & Vanella, K. M. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity, 44(3), 450–462.
Jeljeli, M., Riccio, L. G. C., Doridot, L., Chêne, C., Nicco, C., Chouzenoux, S., Deletang, Q., Allanore, Y., Kavian, N., & Batteux, F. (2019). Trained immunity modulates inflammation-induced fibrosis. Nat Commun, 10(1), 5670.
Wilhelmsen, K., Farrar, K., Tran, A., Khakpour, S., Sundar, S., Prakash, A., Wang, J., Gray, N. S., & Hellman, J. (2015). Extracellular signal-regulated kinase 5 promotes acute cellular and systemic inflammation. Sci Signal, 8(391), ra86.
Ying, Y., Jiang, C., Zhang, M., Ge, S., & Wang, X. (2019). Phloretin protects against cardiac damage and remodeling via restoring SIRT1 and anti-inflammatory effects in the streptozotocin-induced diabetic mouse model. Aging, 11(9), 2822–2835.
Kolaczkowska, E. (2016). The older the faster: aged neutrophils in inflammation. Blood, 128(19), 2280–2282.
Luo, B., Wang, J., Liu, Z., Shen, Z., Shi, R., Liu, Y., Liu, Y., Jiang, M., Wu, Y., & Zhang, Z. (2016). Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat Commun, 7, 12177.
Amor, S., Puentes, F., Baker, D., & Van der Valk, P. (2010). Inflammation in neurodegenerative diseases. Immunology, 129, 154–169.
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., Ferrucci, L., Gilroy, D. W., Fasano, A., Miller, G. W., Miller, A. H., Mantovani, A., Weyand, C. M., Barzilai, N., Goronzy, J. J., Rando, T. A., Effros, R. B., Lucia, A., Kleinstreuer, N., & Slavich, G. M. (2019). Chronic inflammation in the etiology of disease across the life span. Nat Med, 12, 1822–1832.
Greten, F. R., & Grivennikov, S. I. (2019). Inflammation and cancer: triggers, mechanisms, and consequences. Immunity, 51(1), 27–41.
Tremellen, K. (2008). Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update, 14(3), 243–58.
Sena, L. A., & Chandel, N. S. (2012). Physiological roles of mitochondrial reactive oxygen species. Mol Cell, 48(2), 158–67.
Andrisic, L., Dudzik, D., Barbas, C., Milkovic, L., Grune, T., & Zarkovic, N. (2018). Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol, 14, 47–58.
Förstermann, U. (2008). Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med, 5(6), 338–49.
Budak, H., Gonul, N., Ceylan, H., & Kocpinar, E. F. (2014). Impact of long-term Fe3+ toxicity on expression of glutathione system in rat liver. Environ Toxicol Pharmacol, 37(1), 365–70.
Robaczewska, J., Kedziora-Kornatowska, K., Kozakiewicz, M., Zary-Sikorska, E., Pawluk, H., Pawliszak, W., & Kedziora, J. (2016). Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol, 67(3), 331–7.
Prigge, J. R., Coppo, L., Martin, S. S., Ogata, F., Miller, C. G., Bruschwein, M. D., Orlicky, D. J., Shearn, C. T., Kundert, J. A., Lytchier, J., Herr, A. E., Mattsson, Å., Taylor, M. P., Gustafsson, T. N., Arnér, E. S. J., Holmgren, A., & Schmidt, E. E. (2017). Hepatocyte hyperproliferation upon liver-specific co-disruption of Thioredoxin-1, Thioredoxin Reductase-1, and Glutathione Reductase. Cell Rep, 19(13), 2771–2781.
Chong, C. R., Chan, W. P. A., Nguyen, T. H., Liu, S., Procter, N. E. K., Ngo, D. T., Sverdlov, A. L., Chirkov, Y. Y., & Horowitz, J. D. (2014). Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther, 28(4), 347–60.
Lundberg, M., Mattsson, Å., Reiser, K., Holmgren, A., & Curbo, S. (2019). Inhibition of the thioredoxin system by PX-12 (1-methylpropyl 2-imidazolyl disulfide) impedes HIV-1 infection in TZM-bl cells. Sci Rep, 9(1), 5656.
Silva-Adaya, D., Gonsebatt, M. E., & Guevara, J. (2014). Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxid Med Cell Longev, 2014, 590808.
Zhang, J., Li, X., Han, X., Liu, R., & Fang, J. (2017). Targeting the thioredoxin system for cancer therapy. Trends Pharmacol Sci, 38(9), 794–808.
Wu, X. L., Li, X., Li, Y., Kong, L. P., Fang, J. L., Zhou, X. S., Li, M., Jia, J. J., & Bai, J. (2016). The overexpression of Thioredoxin-1 suppressing inflammation induced by methamphetamine in spleen. Drug Alcohol Depend, 159, 66–71.
Hamada, Y., Fujii, H., Kitazawa, R., Yodoi, J., Kitazawa, S., & Fukagawa, M. (2009). Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin-induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone, 44(5), 936–41.
Liu, Z., Jing, Y., Yin, J., Mu, J., Yao, T., & Gao, L. (2013). Downregulation of thioredoxin reductase 1 expression in the substantia nigra pars compacta of Parkinson’s disease mice. Neural Regen Res, 8(35), 3275–83.
Rusetskaya, N. Y., Fedotov, I. V., Koftina, V. A., & Borodulin, V. B. (2019). [Selenium compounds in redox regulation of inflammation and apoptosis]. Biomed Khim, 65(3), 165–179.
Chiang, J. (2014). Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms. Rootstown, OH, USA: Northeast Ohio Medical University.
Kubes, P., & Jenne, C. (2018). Immune responses in the liver. Annu Rev Immunol, 36, 247–277.
Robinson, M. W., Harmon, C., & O’Farrelly, C. (2016). Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol, 3, 267–76.
Sönmez Aydın, F., Hukkamlı, B., & Budak, H. (2021). Coaction of hepatic thioredoxin and glutathione systems in iron overload-induced oxidative stress. J Biochem Mol Toxicol, 35(4), e22704.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys, 82(1), 70–7.
Ceylan, H., Budak, H., Kocpinar, E. F., Baltaci, N. G., & Erdogan, O. (2019). Examining the link between dose-dependent dietary iron intake and Alzheimer’s disease through oxidative stress in the rat cortex. J Trace Elem Med Biol, 56, 198–206.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25(4), 402–8.
Ozgencli, I., Kilic, D., Guller, U., Ciftci, M., Kufrevioglu, O. I., & Budak, H. (2018). A comparison of the inhibitory effects of anti-cancer drugs on thioredoxin reductase and glutathione s-transferase in rat liver. Anticancer Agents Med Chem, 18(14), 2053–2061.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem, 72, 248–54.
Mahmood, T., & Yang, P. C. (2012). Western blot: technique, theory, and trouble shooting. N Am J Med Scİ, 4(9), 429–34.
Güller, P., Budak, H., Şişecioğlu, M., & Çiftci, M. (2020). An in vivo and in vitro comparison of the effects of amoxicillin, gentamicin, and cefazolin sodium antibiotics on the mouse hepatic and renal glutathione reductase enzyme. J Biochem Mol Toxicol, 34(7), e22496.
Ni, J., Zhao, Y., Su, J., Liu, Z., Fang, S., Li, L., Deng, J., & Fan, G. (2020). Toddalolactone protects lipopolysaccharide-induced sepsis and attenuates lipopolysaccharide-induced inflammatory response by modulating HMGB1-NF-κB translocation. Front Pharmacol, 11, 109.
Ahmed, M. B., Islam, S. U. I., & Lee, Y. S. (2020). Decursin negatively regulates LPS-induced upregulation of the TLR4 and JNK signaling stimulated by the expression of PRP4 in vitro. Anim Cells Syst, 24(1), 44–52.
Bieghs, V., & Trautwein, C. (2013). The innate immune response during liver inflammation and metabolic disease. Trends Immunol, 34(9), 446–52.
Ciliberto, G., Arcone, R., Wagner, E. F., & Rüther, U. (1987). Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO J, 6(13), 4017–22.
Rose, S., Melnyk, S., Pavliv, O., Bai, S., Nick, T. G., Frye, R. E., & James, S. J. (2012). Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry, 2(7), e134.
Nguyen, S., Castellanos, K. A., Abraham, A., & Ferrini, M. G. (2021). Reduction of oxidative stress markers in the corpora cavernosa and media of penile dorsal artery in middle-aged rats treated with COMP-4. Int J Impot Res, 33(1), 67–74.
Singh, M., Hamid, A. A., Maurya, A. K., Prakash, O., Khan, F., Kumar, A., Aiyelaagbe, O. O., Negi, A. S., & Bawankule, D. U. (2014). Synthesis of diosgenin analogues as potential anti-inflammatory agents. J Steroid Biochem Mol Biol, 143, 323–33.
Clinton, S. K., Fleet, J. C., Loppnow, H., Salomon, R. N., Clark, B. D., Cannon, J. G., Shaw, A. R., Dinarello, C. A., & Libby, P. (1991). Interleukin-1 gene expression in rabbit vascular tissue in vivo. Am J Pathol, 138(4), 1005–14.
Starr, M. E., Evers, B. M., & Saito, H. (2009). Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci, 64(7), 723–30.
Li, L., Ma, P., Liu, Y., Huang, C., O, W., Tang, F., & Zhang, J. V. (2013). Intermedin attenuates LPS-induced inflammation in the rat testis. PLoS One, 8(6), e65278.
Zitka, O., Skalickova, S., Gumulec, J., Masarik, M., Adam, V., Hubalek, J., Trnkova, L., Kruseova, J., Eckschlager, T., & Kizek, R. (2012). Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett, 4(6), 1247–1253.
Budak, H., Kocpinar, E. F., Gonul, N., Ceylan, H., Erol, H. S., & Erdogan, O. (2014). Stimulation of gene expression and activity of antioxidant-related enzyme in Sprague Dawley rat kidney induced by long-term iron toxicity. Comp Biochem Physiol C Toxicol Pharmacol, 166, 44–50.
Suleyman, H., Dursun, H., Bilici, M., Cadirci, E., Halici, Z., Gulaboglu, M., & Albayrak, F. (2009). Relation of adrenergic receptors, which have roles in gastroprotective and anti-inflammatory effect of adrenal gland hormones, with cyclooxygenase enzyme levels in rats. J Physiol Pharmacol, 60(4), 129–34.
Mahmood, D. F. D., Abderrazak, A., El Hadri, K., Simmet, T., & Rouis, M. (2013). The thioredoxin system as a therapeutic target in human health and disease. Antioxid Redox Signal, 19(11), 1266–303.
Arnér, E. S. J., & Holmgren, A. (2006). The thioredoxin system in cancer. Semin Cancer Biol, 16(6), 420–6.
Bian, M., Fan, R., Zhao, S., & Liu, W. (2019). Targeting the thioredoxin system as a strategy for cancer therapy. J Med Chem, 62(16), 7309–7321.
Lu, J., & Holmgren, A. (2014). The thioredoxin antioxidant system. Free Radic Biol Med, 66, 75–87.
Watson, W. H., Yang, X., Choi, Y. E., Jones, D. P., & Kehrer, J. P. (2004). Thioredoxin and its role in toxicology. Toxicol Sci, 78(1), 3–14.
Matsui, M., Oshima, M., Oshima, H., Takaku, K., Maruyama, T., Yodoi, J., & Taketo, M. M. (1996). Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol, 178(1), 179–85.
Nonn, L., Williams, R. R., Erickson, R. P., & Powis, G. (2003). The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol, 23(3), 916–22.
Lee, S., Kim, S. M., & Lee, R. T. (2013). Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid Redox Signal, 18(10), 1165–207.
Kamimoto, Y., Sugiyama, T., Kihira, T., Zhang, L., Murabayashi, N., Umekawa, T., Nagao, K., Ma, N., Toyoda, N., Yodoi, J., & Sagawa, N. (2010). Transgenic mice overproducing human thioredoxin-1, an antioxidative and anti-apoptotic protein, prevents diabetic embryopathy. Diabetologia, 53(9), 2046–55.
Salmon AB, Flores LC, Li Y, Remmen HV, Richardson A, Ikeno Y. (2012). Reduction of glucose intolerance with high-fat feeding is associated with anti-inflammatory effects of thioredoxin 1 overexpression in mice. Pathobiol Aging Age Relat Dis, 2.
Okuyama, H., Nakamura, H., Shimahara, Y., Araya, S., Kawada, N., Yamaoka, Y., & Yodoi, J. (2003). Overexpression of thioredoxin prevents acute hepatitis caused by thioacetamide or lipopolysaccharide in mice. Hepatology, 37(5), 1015–25.
Okuyama, H., Nakamura, H., Shimahara, Y., Uyama, N., Kwon, Y. W., Kawada, N., Yamaoka, Y., & Yodoi, J. (2005). Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J Hepatol, 42(1), 117–23.
Shalev, A. (2014). Minireview: Thioredoxin-interacting protein: regulation and function in the pancreatic β-cell. Mol Endocrinol, 28(8), 1211–20.
Alhawiti, N. M., Mahri, S. A., Aziz, M. A., Malik, S. S., & Mohammad, S. (2017). TXNIP in metabolic regulation: physiological role and therapeutic outlook. Curr Drug Targets, 18(9), 1095–1103.
Jia, J., Geng, W., Wang, Z., Chen, L., & Zeng, X. (2019). The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol, 84(3), 453–470.
Becker, K., Gromer, S., Schirmer, R. H., & Müller, S. (2000). Thioredoxin reductase as a pathophysiological factor and drug target. Eur J Biochem, 267(20), 6118–25.
Grogan, T. M., Fenoglio-Prieser, C., Zeheb, R., Bellamy, W., Frutiger, Y., Vela, E., Stemmerman, G., Macdonald, J., Richter, L., Gallegos, A., & Powis, G. (2000). Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol, 31(4), 475–81.
Kakolyris, S., Giatromanolaki, A., Koukourakis, M., Powis, G., Souglakos, J., Sivridis, E., Georgoulias, V., Gatter, K. C., & Harris, A. L. (2001). Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res, 7(10), 3087–91.
Cadenas, C., Franckenstein, D., Schmidt, M., Gehrmann, M., Hermes, M., Geppert, B., Schormann, W., Maccoux, L. J., Schug, M., Schumann, A., Wilhelm, C., Freis, E., Ickstadt, K., Rahnenführer, J., Baumbach, J. I., Sickmann, A., & Hengstler, J. G. (2010). Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Res, 12(3), R44.
Bhatia, M., Lovitt, C. J., Raninga, P. V., Avery, V. M., Di Trapani, G., & Tonissen, K. F. (2016). Expression of the thioredoxin system in an in vivo-like cancer cell environment upon auranofin treatment. Eur J Cell Biol, 95(10), 378–388.
Lei, H., Wang, G., Zhang, J., & Han, Q. (2018). Inhibiting TrxR suppresses liver cancer by inducing apoptosis and eliciting potent antitumor immunity. Oncol Rep, 40(6), 3447–3457.
Zhou, J., & Chng, W. J. (2012). Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion, 13(3), 163–9.
Sheth, S. S., Bodnar, J. S., Ghazalpour, A., Thipphavong, C. K., Tsutsumi, S., Tward, A. D., Demant, P., Kodama, T., Aburatani, H., & Lusis, A. J. (2006). Hepatocellular carcinoma in Txnip-deficient mice. Oncogene, 25(25), 3528–36.
Minn, A. H., Hafele, C., & Shalev, A. (2005). Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology, 146(5), 2397–405.
Alhawiti, N. M., Al Mahri, S., Aziz, M. A., Malik, S. S., & Mohammad, S. (2017). TXNIP in metabolic regulation: physiological role and therapeutic outlook. Curr Drug Targets, 18(9), 1095–1103.
Conterato, G. M. M., Quatrin, A., Somacal, S., Ruviaro, A. R., Vicentini, J., Augusti, P. R., Sobieski, R., Figueiredo, C., Dos Santos, C. M. M., Pereira, T. C., Bogo, M. R., Flores, E. M. M., & Emanuelli, T. (2014). Acute exposure to low lead levels and its implications on the activity and expression of cytosolic thioredoxin reductase in the kidney. Basic Clin Pharmacol Toxicol, 114(6), 476–84.
Kasuno, K., Nakamura, H., Ono, T., Muso, E., & Yodoi, J. (2003). Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney InT, 64(4), 1273–82.
Hou, X., Yang, S., & Yin, J. (2019). Blocking the REDD1/TXNIP axis ameliorates LPS-induced vascular endothelial cell injury through repressing oxidative stress and apoptosis. Am J Physiol Cell Physiol, 316(1), C104–C110.
De Marinis, Y., Cai, M., Bompada, P., Atac, D., Kotova, O., Johansson, M. E., Garcia-Vaz, E., Gomez, M. F., Laakso, M., & Groop, L. (2016). Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney Int, 89(2), 342–53.
He, Q., Li, Y., Zhang, W., Chen, J., Deng, W., Liu, Q., Liu, Y., & Liu, D. (2021). Role and mechanism of TXNIP in ageing-related renal fibrosis. Mech Ageing Dev, 196, 111475.
Hamada, Y., & Fukagawa (2007). A possible role of thioredoxin interacting protein in the pathogenesis of streptozotocin-induced diabetic nephropathy. M Kobe J Med Sci, 53(1-2), 53–61.
Chen, Y., Ning, J., Cao, W., Wang, S., Du, T., Jiang, J., Feng, X., & Zhang, B. (2020). Research progress of TXNIP as a tumor suppressor gene participating in the metabolic reprogramming and oxidative stress of cancer cells in various cancers. Front Oncol, 10, 568574.
Huang, C., Zhang, Y., Kelly, D. J., Tan, C. Y., Gill, A., Cheng, D., Braet, F., Park, J. S., Sue, C. M., Pollock, C. A., & Chen, X. M. (2016). Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci Rep, 6, 29196.
Qayyum, N., Haseeb, M., Kim, M. S., & Choi, S. (2021). Role of Thioredoxin-interacting protein in diseases and its therapeutic outlook. Int J Mol Sci, 22(5), 2754.
Cebula, M., Schmidt, E. E., & Arnér, E. S. J. (2015). TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal, 23(10), 823–853.
Branco, V., Coppo, L., Solá, S., Lu, J., Rodrigues, C. M. P., Holmgren, A., & Carvalho, C. (2017). Impaired cross-talk between the thioredoxin and glutathione systems is related to ASK-1-mediated apoptosis in neuronal cells exposed to mercury. Redox Biol, 13, 278–287.
Li, Y., Zhang, Y., Gao, Y., Zhang, W., Cui, X., Liu, J., & Wei, Y. (2018). Arsenic induces Thioredoxin 1 and Apoptosis in human liver HHL-5 Cells. Biol Trace Elem Res, 181(2), 234–241.
Ratnayake, S., Dias, I. H. K., Lattman, E., & Griffiths, H. R. (2013). Stabilising cysteinyl thiol oxidation and nitrosation for proteomic analysis. J Proteomics, 92, 160–70.
Ungerstedt, J., Du, Y., Zhang, H., Nair, D., & Holmgren, A. (2012). In vivo redox state of human thioredoxin and redox shift by the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA). Free Radic Biol Med, 53(11), 2002–7.
Wright, D. E., Altaany, Z., Bi, Y., Alperstein, Z., & O’Donoghue, P. (2018). Acetylation regulates thioredoxin reductase oligomerization and activity. Antioxid Redox Signal, 29(4), 377–388.
Ooi, K. K., Yeo, C. I., Ang, K. P., Akim, A. M., Cheah, Y. K., Halim, S. N., Seng, H. L., & Tiekink, E. R. (2015). Phosphanegold(I) thiolates, Ph3PAu[SC(OR)=NC6H4Me-4] for R = Me, Et and iPr, induce apoptosis, cell cycle arrest and inhibit cell invasion of HT-29 colon cancer cells through modulation of the nuclear factor-κB activation pathway and ubiquitination. J Biol Inorg Chem, 20(5), 855–73..
Engelman, R., Weisman-Shomer, P., Ziv, T., Xu, J., Arnér, E. S. J., & Benhar (2013). Multilevel regulation of 2-Cys peroxiredoxin reaction cycle by S-nitrosylation. M J Biol Chem, 288(16), 11312–24.
Qin, H., Liang, W., Xu, Z., Ye, F., Li, X., & Zhong, L. (2015). Mechanistic insights into the inhibitory effects of palmitoylation on cytosolic thioredoxin reductase and thioredoxin. Biochimie, 110, 25–35.
Yuan, Y., Jiao, X., Lau, W. B., Wang, Y., Christopher, T. A., Lopez, B. L., Ramachandrarao, S. P., Tao, L., & Ma, X. L. (2010). Thioredoxin glycation: A novel posttranslational modification that inhibits its antioxidant and organ protective actions. Free Radic Biol Med, 49(3), 332–8.
Sheth, S. S., Castellani, L. W., Chari, S., Wagg, C., Thipphavong, C. K., Bodnar, J. S., Tontonoz, P., Attie, A. D., Lopaschuk, G. D., & Lusis, A. J. (2005). Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res, 46(1), 123–34.
Yoshioka, J., Imahashi, K., Gabel, S. A., Chutkow, W. A., Burds, A. A., Gannon, J., Schulze, P. C., MacGillivray, C., London, R. E., Murphy, E., & Lee, R. T. (2007). Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res, 101(12), 1328–38.
Hui, T. Y., Sheth, S. S., Diffley, J. M., Potter, D. W., Lusis, A. J., Attie, A. D., & Davis, R. A. (2004). Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem, 279(23), 24387–93.
Lian, D., Dai, L., Xie, Z., Zhou, X., Liu, X., Zhang, Y., Huang, Y., & Chen, Y. (2018). Periodontal ligament fibroblasts migration injury via ROS/TXNIP/Nlrp3 inflammasome pathway with Porphyromonas gingivalis lipopolysaccharide. Mol Immunol, 103, 209–219.
Jirillo, E., Caccavo, D., Magrone, T., Piccigallo, E., Amati, L., Lembo, A., Kalis, C., & Gumenscheimer, M. (2002). The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res, 8(5), 319–27.
Park, Y. S., Fujiwara, N., Koh, Y. H., Miyamoto, Y., Suzuki, K., Honke, K., & Taniguchi, N. (2002). Induction of thioredoxin reductase gene expression by peroxynitrite in human umbilical vein endothelial cells. Biol Chem, 383(3-4), 683–91.
Author contributions
Each of the authors contributed to the paper. The preparation of the materials, the experiments, and the collection and analysis of the data were done by BH, BD, FSA, and HB. The research text was written by BH and HB. Furthermore, all authors confirmed the recent version of the article.
Funding
This work was financed by the Scientific and Technological Research Council of Türkiye (TÜBİTAK) (Project No. 114Z277) and the Scientific Research Projects Coordination Commission of Atatürk University (Project no. PRJ2016/151). Furthermore, one author (Feyza Sönmez Aydın, Ph.D.) was supported by the Council of Higher Education (CoHE) Scientific and Technological Research of Türkiye (TÜBİTAK) 2211- A Ph.D. Scholarship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Humans were not used in the experiments in this study. Experiments using animals were carried out following the Laboratory Animal Care and Use Guidelines after approval was obtained from the local animal care committee of Atatürk University (Protocol no: 55885869-381).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hukkamlı, B., Dağdelen, B., Sönmez Aydın, F. et al. Comparison of the efficacy of the mouse hepatic and renal antioxidant systems against inflammation-induced oxidative stress. Cell Biochem Biophys 81, 299–311 (2023). https://doi.org/10.1007/s12013-023-01126-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01126-3