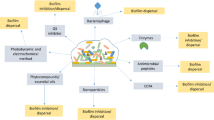
Abstract
Helminths are considered a significant threat to the livestock industry, as they cause substantial economic losses in small and large ruminant farming. Their morbidity and mortality rates are also increasing day by day as they have zoonotic importance. Anthelmintic drugs have been used for controlling these parasites; unfortunately, due to the development of resistance of these drugs in helminths (parasites), especially in three major classes like benzimidazoles, nicotinic agonists, and macrocyclic lactones, their use is becoming very low. Although new anthelmintics are being developed, the process is time-consuming and costly. As a result, nanoparticles are being explored as an alternative to anthelmintics. Nanoparticles enhance drug effectiveness, drug delivery, and target specificity and have no resistance against parasites. Different types of nanoparticles are used, such as organic (chitosan) and inorganic (gold, silver, zinc oxide, iron oxide, and nickel oxide). One of them, silver nanoparticles (AgNPs), has unique properties in various fields, especially parasitology. AgNPs are synthesized from three primary methods: physical, chemical, and biological. Their primary mechanism of action is causing stress through the production of ROS that destroys cells, organs, proteins, and DNA parasites. The present review is about AgNPs, their mode of action, and their role in controlling anthelmintic resistance against small and large ruminants.


Similar content being viewed by others
References
Mavrot F, Hertzberg H, Torgerson P (2015) Effect of gastro-intestinal nematode infection on sheep performance: a systematic review and meta-analysis. Parasit Vectors 8:1–11. https://doi.org/10.1186/s13071-015-1164-z
Charlier J, Rinaldi L, Musella V, Ploeger HW et al (2020) Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med 182:1–12. https://doi.org/10.1016/j.prevetmed.2020.105103
Vineer HR, Morgan ER, Hertzberg H et al (2020) Increasing importance of anthelmintic resistance in European livestock: creation and meta-analysis of an open database. Parasite 27:1–69. https://doi.org/10.1051/parasite/2020062
Tumusiime M, Ndayisenga F, Ntampaka P (2022) Prevalence of gastrointestinal nematodes, cestodes, and protozoans of goats in Nyagatare District, Rwanda. Vet Med Res Rep 13:339–349. https://doi.org/10.2147/VMRR.S389336
Charlier J, van der Voort M, Kenyon F, Skuce P, Vercruysse J (2014) Chasing helminths and their economic impact on farmed ruminants. Trend Parasitol 30:361–367. https://doi.org/10.1016/j.pt.2014.04.009
Rehman A, Abidi SMA (2022) Livestock health: current status of helminth infections and their control for sustainable development. Advances in animal experimentation and modeling 365–378 https://doi.org/10.1016/B978-0-323-90583-1.00029-5
Qamar W, Zaman MA, Faheem M, Ahmed I, Ali K, Qamar MF et al (2022) Molecular confirmation and genetic characterization of Haemonchus contortus isolates at the nuclear ribosomal ITS2 region: first update from Jhang Region of Pakistan. Pak Vet J 42:251–255. https://doi.org/10.29261/pakvetj/2021.071
Mahmoud HY, Ali AAA, Khalil AM, Amin YA, Ali AO (2022) The infection rate of Fasciola and Anaplasma in cattle and buffaloes in Qena, Egypt. Int J Vet Sci 11:308–314
Mahmood Q, Younus M, Sadiq S, Iqbal S, Idrees A, Khan S, Zia R (2022) Prevalence and associated risk factors of Cystic echinococcosis in food animals–a neglected and prevailing zoonosis. Pak Vet J 42:59–64. https://doi.org/10.29261/pakvetj/2022.008
Beleckė A, Kupčinskas T, Stadalienė I, Höglund J, Thamsborg SM, Stuen S, Petkevičius S (2021) Anthelmintic resistance in small ruminants in the Nordic-Baltic region. Acta Vet Scand 63:1–7. https://doi.org/10.1186/s13028-021-00583-1
Kapo N, Omeragić J, Tandir F, Mujezinović I, Smajlović A, Šaljić E (2022) Anthelmintic resistance in gastrointestinal nematodes of ruminants. Proceedings of Socratic Lectures 7:64–67. https://doi.org/10.55295/PSL.2022
Amarante AF (2014) Sustainable worm control practices in South America. Small Rumin Res 118:56–62. https://doi.org/10.1016/j.smallrumres.2013.12.016
JH, das Neves, N, Carvalho, L, Rinaldi, G, Cringoli, AF, Amarante (2014) Diagnosis of anthelmintic resistance in cattle in Brazil: a comparison of different methodologies. Vet Parasitol 206:216–226. https://doi.org/10.1016/j.vetpar.2014.10.015
Nawaz M, Zhou JL, Shamim A et al (2022) Antiparasitic activity of plants extract against gastrointestinal nematodes and Rhipicephalus microplus. Int J Vet Sci 11:474–478
Al-Saeed FA, Bamarni SSI, Iqbal KJ et al (2023) In vitro anthelmintic efficacy of haloxylon salicornicum leaves extract using adult Heamonchus contortus worms. Pak Vet J 43:1–6. https://doi.org/10.29261/pakvetj/2022.091
Velázquez-Antunez J, Olivares-Perez J, Olmedo-Juárez A, Rojas-Hernandez S, Villa-Mancera A, RomeroRosales T (2023) Biological activity of the secondary compounds of Guazuma ulmifolia leaves to inhibit the hatching of eggs of Haemonchus contortus. Pak Vet J 43:55–60. https://doi.org/10.29261/pakvetj/2022.075
Bisset SA, Knight JS, Bouchet C (2014) A multiplex PCR-based method to identify strongylid parasite larvae 400 recovered from ovine faecal cultures and/or pasture samples. Vet Parasitol 200:117–127. https://doi.org/10.1016/j.vetpar.2013.12.002
Fairweather I, Brennan GP, Hanna REB, Robinson MW, Skuce PJ (2020) Drug resistance in liver flukes. Int J Parasitol 12:39–59. https://doi.org/10.1016/j.ijpddr.2019.11.003
Prueksapanich P, Piyachaturawat P, Aumpansub P, Ridtitid W, Chaiteerakij R, Rerknimitr R (2018) Liver fluke-associated biliary tract cancer. Gut liver 17:236–245. https://doi.org/10.5009/gnl17102
Mostafa W, Abdel-Rady A, El-Dakroury MF, Felefel W (2023) Field trials to evaluate five fasciolicides against natural liver fluke infection in cattle and sheep in Egypt. Int J Vet Sci 12:76–81
Elsaid FG, Garijo Toledo MM, Gentile A, Gul RA, Rashid M (2023) Fasciolosis recent update in vaccines development and their efficacy Pak Vet J 43
Ramos F, Portella LP, Rodrigues FS et al (2016) Anthelmintic resistance in gastrointestinal nematodes of beef cattle in the state of Rio Grande do Sul, Brazil. Int J Parasitol Drugs Drug Resist 6:93–101. https://doi.org/10.1016/j.ijpddr.2016.02.002
Hamid L, Alsayari A, Tak H, Mir SA, Almoyad MAA, Wahab S, Bader GN (2023) An insight into the global problem of gastrointestinal helminth infections amongst livestock: Does nanotechnology provide an alternative? Agriculture 13:1–19. https://doi.org/10.3390/agriculture13071359
Batool S, Munir F, Sindhu ZUD, Abbas RZ et al (2023) In vitro anthelmintic activity of Azadirachta indica (Neem) and Melia azedarach (Bakain) essential oils and their silver nanoparticles against Haemonchus contortus. Agrobiol Rec 11:6–12. https://doi.org/10.47278/journal.abr/2023.002
Beyene T (2016) Veterinary drug residues in food-animal products: its risk factors and potential effects on public health. J Vet Sci Technol 7:1–7. https://doi.org/10.4172/2157-7579.1000285
Ahuir-Baraja AE, Cibot F, Llobat L, Garijo MM (2021) Anthelmintic resistance: is a solution possible? Exp Parasitol 230:1–7. https://doi.org/10.1016/j.exppara.2021.108169
Vineer HR (2020) What modeling parasites, transmission, and resistance can teach us. Vet Clin North Am Food Anim Pract 36:145–158. https://doi.org/10.1016/j.cvfa.2019.11.002
Charlier J, Bartley DJ, Sotiraki S et al (2022) Anthelmintic resistance in ruminants: challenges and solutions. Adv parasitol 115:171–227. https://doi.org/10.1016/bs.apar.2021.12.002
Baudinette E, O’Handley R, Trengove C (2022) Anthelmintic resistance of gastrointestinal nematodes in goats: a systematic review and meta-analysis. Vet Parasitol 312:1–15. https://doi.org/10.1016/j.vetpar.2022.109809
Degla LH, Kuiseu J, Olounlade PA et al (2022) Use of medicinal plants as alternative for the control of intestinal parasitosis: assessment and perspectives. Agrobiol Rec 7:1–9. https://doi.org/10.47278/journal.abr/2021.011
Bartley DJ, Hamer K, Andrews L, Sargison ND, Morrison AA (2019) Multigeneric resistance to monepantel on a UK sheep farm. Vet Parasitol 276:1–3. https://doi.org/10.1016/j.vpoa.2019.100003
Geurden T, Hoste H, Jacquiet P et al (2014) Anthelmintic resistance and multidrug resistance in sheep gastro-intestinal nematodes in France, Greece and Italy. Vet Parasitol 201:59–66. https://doi.org/10.1016/j.vetpar.2014.01.016
Martínez-Valladares M, Martínez-Pérez JM, Robles-Pérez D et al (2013) The present status of anthelmintic resistance in gastrointestinal nematode infections of sheep in the northwest of Spain by in vivo and in vitro techniques. Vet Parasitol 191:177–181. https://doi.org/10.1016/j.vetpar.2012.08.009
Kelleher AC, Good B, de Waal T, Keane OM (2020) Anthelmintic resistance among gastrointestinal nematodes of cattle on dairy calf to beef farms in Ireland. Irish Vet J 73:1–8. https://doi.org/10.1186/s13620-020-00167-x
Zanzani SA, Gazzonis AL, Di Cerbo A, Varady M, Manfredi MT (2014) Gastrointestinal nematodes of dairy goats, anthelmintic resistance and practices of parasite control in Northern Italy. BMC Vet Research 10:1–10. https://doi.org/10.1186/1746-6148-10-114
El-Hamaky AM, Hassan AA, Wahba AK, El MM (2023) Influence of copper and zinc nanoparticles on genotyping characterizations of multi-drug resistance genes for some calf pathogens. Int J Vet Sci 12:309–317. https://doi.org/10.47278/journal.ijvs/2022.195
Murugan K, Anitha J, Suresh U, Rajaganesh R et al (2017) Chitosan-fabricated Ag nanoparticles and larvivorous fishes: a novel route to control the coastal malaria vector Anopheles sundaicus? Hydrobiologia 797:335–350. https://doi.org/10.1007/s10750-017-3196-1
Benelli G (2018) Gold nanoparticles–against parasites and insect vectors. Acta Trop 178:73–80. https://doi.org/10.1016/j.actatropica.2017.10.021
Shnawa BH, Jalil PJ, Aspoukeh P, Mohammed DA, Biro DM (2022) Protoscolicidal and biocompatibility properties of biologically fabricated zinc oxide nanoparticles using Ziziphus spina-christi Leaves. Pak Vet J 42:517–525. https://doi.org/10.29261/pakvetj/2022.058
Abd El Rahim SA, Arafa MM, Abdelhamid HY, Mohamed AESA (2023) Effect of zinc oxide nanoparticles on some biochemical parameters and body weight in Barki fattening lambs. SVU- Int J Vet Sci 6:88–103. https://doi.org/10.21608/svu.2023.191602.1258
Aqib AI, Akram K, Majeed H, Murtaza M, Muneer A, Alsayeqh AF (2022) Resistance modulation of dairy milk borne Streptococcus agalactiae and Klebsiella pneumoniae through metallic oxide nanoparticles. Pak Vet J 42:424–428. https://doi.org/10.29261/pakvetj/2022.052
Goel V, Kaur P, Singla LD, Choudhury D (2020) Biomedical evaluation of Lansium parasiticum extract-protected silver nanoparticles against Haemonchus contortus, a parasitic worm. Front Mol Biosci 7:1–14. https://doi.org/10.3389/fmolb.2020.595646
Silva LP, Silveira AP, Bonatto CC, Reis IG, Milreu PV (2017) Elsevier 577–596. https://doi.org/10.1016/B978-0-323-46152-8.00026-3
Bruna T, Maldonado-Bravo F, Jara P, Caro N (2021) Silver nanoparticles and their antibacterial applications. Int J Mol Sci 22:7202. https://doi.org/10.3390/ijms22137202
Tran QH, Le AT (2013) Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives (Adv. Nat. Sci: Nanosci. Nanotechnol. 4 033001). Adv Net Sci Nanosci Nanotechnol 9 https://doi.org/10.1088/2043-6254/aad12b
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17:1–34. https://doi.org/10.3390/ijms17091534
Yaqoob AA, Umar K, Ibrahim MNM (2020) Silver nanoparticles: various methods of synthesis, size affecting factors and their potential applications–a review. Appl Nanosci 10:1369–1378. https://doi.org/10.1007/s13204-020-01318-w
Cho KY, Moon JY, Lee YW et al (2003) Preparation of self-assembled silk sericin nanoparticles. Int J Biol Macromol 32:36–42. https://doi.org/10.1016/S0141-8130(03)00023-0
Singh A, Hede S, Sastry M (2007) Spider silk as an active scaffold in the assembly of gold nanoparticles and application of the gold–silk bioconjugate in vapor sensing. Small 3:466–473. https://doi.org/10.1002/smll.200600413
Tokarek K, Hueso JL, Kuśtrowski P, Stochel G, Kyzioł A (2013) Green synthesis of chitosan-stabilized copper nanoparticles. Eur J Inorg Chem 2013:4940–4947. https://doi.org/10.1002/ejic.201300594
Aramwit P, Bang N, Ratanavaraporn J, Ekgasit S (2014) Green synthesis of silk sericin-capped silver nanoparticles and their potent anti-bacterial activity. Nanoscale Res Lett 9:1–7. https://doi.org/10.1186/1556-276X-9-79
Balavandy SK, Shameli K, Abidin ZZ (2015) Rapid and green synthesis of silver nanoparticles via sodium alginate media. Int J Electrochem Sci 10:486–497. https://doi.org/10.1016/S1452-3981(23)05007-1
Han L, Kim YS, Cho S, Park Y (2013) Invertebrate water extracts as biocompatible reducing agents for the green synthesis of gold and silver nanoparticles. Nat Prod Commun 8:1149–1152. https://doi.org/10.1177/1934578X1300800830
Schmalz G, Hickel R, van Landuyt KL, Reichl FX (2017) Nanoparticles in dentistry. Dent Mater 33:1298–1314. https://doi.org/10.1016/j.dental.2017.08.193
Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H (2020) Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics 10:8996–9031 (https://www.thno.org/v10p8996.htm)
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385–406
Zhang P, Gong J, Jiang Y, Long Y, Lei W, Gao X, Guo D (2023) Application of silver nanoparticles in parasite treatment. Pharmaceutics 15:1–17. https://doi.org/10.3390/pharmaceutics15071783
Syed A, Saraswati S, Kundu GC, Ahmad A (2013) Biological synthesis of silver nanoparticles using the fungus Humicola sp and evaluation of their cytoxicity using normal and cancer cell lines. Spectrochim Acta A Mol Biomol Spectrosc 114:144–147. https://doi.org/10.1016/j.saa.2013.05.030
Takeshima T, Tada Y, Sakaguchi N, Watari F, Fugetsu B (2015) DNA/Ag nanoparticles as antibacterial agents against gram-negative bacteria. Nanomaterials 5:284–297. https://doi.org/10.3390/nano5010284
Alves MF, Murray PG (2022) Biological synthesis of monodisperse uniform-size silver nanoparticles (AgNPs) by fungal cell-free extracts at elevated temperature and pH. J Fungi 8:1–14. https://doi.org/10.3390/jof8050439
Alves MF, Paschoal ACC, Klimeck TDMF, Kuligovski C, Marcon BH, de Aguiar AM, Murray PG (2022) Biological synthesis of low cytotoxicity silver nanoparticles (AgNPs) by the fungus chaetomium thermophilum—sustainable nanotechnology. J Fungi 8:1–15. https://doi.org/10.3390/jof8060605
Okonkwo IF, Achilike KM (2022) Comparative assessment of antimicrobial activities of Allium cepa (onions) extract. Agrobiol Rec 9:73–9. https://doi.org/10.47278/journal.abr/2022.012
Aritonang HF, Koleangan H, Wuntu AD (2019) Synthesis of silver nanoparticles using aqueous extract of medicinal plants’(Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. Int J microbiol 2019:1–8. https://doi.org/10.1155/2019/8642303
Ibrahim S, Ahmad Z, Manzoor MZ, Mujahid M, Faheem Z, Adnan A (2021) Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci Rep 11:770. https://doi.org/10.1038/s41598-020-80805-0
Wang Y, Yan C, Li C, Z, Lu C, Ma, Y, Yan, Y, Zhang (2018) Charge transfer tuned by the surrounding dielectrics in TiO2-Ag composite arrays. Nanomaterials 8:1019–9031. https://doi.org/10.3390/nano8121019
Das RK, Brar SK, Verma M, Surampalli RY (2016) Biological synthesis of metallic nanoparticles: making sense of greenness versus unforeseen arbitraries. J Hazard Toxic Radioact Waste 20:04015015. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000294
Singh R, Sahu SK, Thangaraj M (2014) Biosynthesis of silver nanoparticles by marine invertebrate (polychaete) and assessment of its efficacy against human pathogens. J Nanoparticles 2014:1–7. https://doi.org/10.1155/2014/718240
Mofidfar M, Kim ES, Larkin EL, Long L et al (2019) Antimicrobial activity of silver containing crosslinked poly (acrylic acid) fibers. Micromachines 10:1–12. https://doi.org/10.3390/mi10120829
Quintero-Quiroz C, Acevedo N, Zapata-Giraldo J, Botero LE et al (2019) Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater Res 23:1–15. https://doi.org/10.1186/s40824-019-0173-y
Liu M, Fang F, Song X, Yu F et al (2016) The first visually observable three-mode antibiotic switch and its relative 3D printing assisted applications. J Mater Chem B 4:2544–2547. https://doi.org/10.1039/C6TB00576D
Zaarour M, El Roz M, Dong B, Retoux R et al (2014) Photochemical preparation of silver nanoparticles supported on zeolite crystals. Langmuir 30:6250–6256. https://doi.org/10.1021/la5006743
Jovanović Ž, Radosavljević A, Stojkovska J, Nikolić B, Obradovic B, Kačarević-Popović Z, Mišković-Stanković V (2014) Silver/poly (N-vinyl-2-pyrrolidone) hydrogel nanocomposites obtained by electrochemical synthesis of silver nanoparticles inside the polymer hydrogel aimed for biomedical applications. Polym Compos 35:217–226. https://doi.org/10.1002/pc.22653
Zhao X, Xia Y, Li Q, Ma X, Quan F, Geng C, Han Z (2014) Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf A Physicochem Eng 444:180–188. https://doi.org/10.1016/j.colsurfa.2013.12.008
Calderón-Jiménez B, Montoro Bustos AR, Pereira Reyes R, Paniagua SA, Vega-Baudrit JR (2022) Novel pathway for the sonochemical synthesis of silver nanoparticles with near-spherical shape and high stability in aqueous media. Sci Rep 12:882. https://doi.org/10.1038/s41598-022-04921-9
Kaabipour S, Hemmati S (2021) A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J Nanotechnol 12:102–136. https://doi.org/10.3762/bjnano.12.9
Xing T, Sunarso J, Yang W, Yin Y, Glushenkov AM, Li LH, Chen Y (2013) Ball milling: a green mechanochemical approach for synthesis of nitrogen doped carbon nanoparticles. Nanoscale 5:7970–7976. https://doi.org/10.1039/C3NR02328A
Ullah M, Ali M, Hamid S (2014) Structure-controlled nanomaterial synthesis using surfactant-assisted ball milling- a review. Curr Nanosci 10:344–354. https://doi.org/10.2174/15734137113096660114
Thamizharasan S, Saravanan NA (2017) Nanosization of drug biomaterials and its solubility enhancement by high energy ball milling J Nanosci Technol 237–239
Tavakoli A, Sohrabi M, Kargari A (2007) A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem Pap 61:151–170. https://doi.org/10.2478/s11696-007-0014-7
Kruis FE, Fissan H, Rellinghaus B (2000) Sintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticles. Mater Sci Eng B 69:329–334. https://doi.org/10.1016/S0921-5107(99)00298-6
Rafique M, Sadaf I, Rafique MS, Tahir MB (2017) A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45:1272–1291. https://doi.org/10.1080/21691401.2016.1241792
Magnusson MH, Deppert K, Malm JO, Bovin JO, Samuelson L (1999) Gold nanoparticles: production, reshaping, and thermal charging. J Nanopart Res 1:243–251. https://doi.org/10.1023/A:1010012802415
Natsuki J, Natsuki T, Hashimoto Y (2015) A review of silver nanoparticles: synthesis methods, properties and applications. Int J Mater Sci Appl 4:325–332. https://doi.org/10.11648/j.ijmsa.20150405.17
Amendola V, Meneghetti M (2009) Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys Chem Chem Phys 11:3805–3821. https://doi.org/10.1039/B900654K
Amendola V, Meneghetti M (2013) What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys Chem Chem Phys 15:3027–3046. https://doi.org/10.1039/C2CP42895D
Sportelli MC, Izzi M, Volpe A, Clemente M et al (2018) The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics 67:1–28. https://doi.org/10.3390/antibiotics7030067
Okuyama K, Lenggoro IW (2003) Preparation of nanoparticles via spray route. Chem Eng Sci 58:537–547. https://doi.org/10.1016/S0009-2509(02)00578-X
Ghaffarian HR, Saiedi M, Sayyadnejad MA, Rashidi AM (2011) Synthesis of ZnO nanoparticles by spray pyrolysis method. Iran J Chem Chem Eng 30:1–16
Keskar M, Sabatini C, Cheng C, Swihart MT (2019) Synthesis and characterization of silver nanoparticle-loaded amorphous calcium phosphate microspheres for dental applications. Nanoscale Adv 1:627–635. https://doi.org/10.1039/C8NA00281A
Ashkarran AA (2010) A novel method for synthesis of colloidal silver nanoparticles by arc discharge in liquid. Curr Appl Phys 10:1442–1447. https://doi.org/10.1016/j.cap.2010.05.010
Zhang H, Zou G, Liu L, Tong H, Li Y, Bai H, Wu A (2017) Synthesis of silver nanoparticles using large-area arc discharge and its application in electronic packaging. J Mater Sci 52:3375–3387. https://doi.org/10.1007/s10853-016-0626-9
Tseng K-H, Chou C-J, Liu T-C, Tien D-C, Wu T-C, Stobinski L (2018) Interactive relationship between silver ions and silver nanoparticles with PVA prepared by the submerged arc discharge method. Adv Mater Sci Eng 7:1–19. https://doi.org/10.1155/2018/3240959
Tseng KH, Chou CJ, Liu TC, Tien DC, Chang CY, Stobinski L (2018) Relationship between Ag nanoparticles and Ag ions prepared by arc discharge method. Nanotechnol Rev 7:1–9. https://doi.org/10.1515/ntrev-2017-0167
Khan M, Shaik MR, Adil SF, Khan ST, Al-Warthan A, Siddiqui MRH, Tremel W (2018) Plant extracts as green reductants for the synthesis of silver nanoparticles: lessons from chemical synthesis. Dalton Trans 47:11988–12010. https://doi.org/10.1039/C8DT01152D
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145:83–96. https://doi.org/10.1016/j.cis.2008.09.002
Hussain I, Singh NB, Singh A, Singh H, Singh SC (2016) Green synthesis of nanoparticles and its potential application. Biotechnol Lett 38:545–560. https://doi.org/10.1007/s10529-015-2026-7
Parveen K, Banse V, Ledwani L (2016). Green synthesis of nanoparticles: their advantages and disadvantages AIP Conf Proc 1724 https://doi.org/10.1063/1.4945168
Kumar P, Singh PK, Hussain M, Kumar Das A (2016) Synthesis of silver metal nanoparticles through electric arc discharge method: a review. Adv Sci Lett 22:3–7. https://doi.org/10.1166/asl.2016.6772
Shivaji S, Madhu S, Singh S (2011) Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem 46:1800–1807. https://doi.org/10.1016/j.procbio.2011.06.008
Sastry M, Ahmad A, Khan MI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr sci 85:162–170
Korbekandi H, Mohseni S, Mardani Jouneghani R, Pourhossein M, Iravani S (2016) Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artificial cells Nanomed Biotechnol 44:235–239. https://doi.org/10.3109/21691401.2014.937870
Fernández JG, Fernández-Baldo MA, Berni E, Camí G, Durán N, Raba J, Sanz MI (2016) Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem 51:1306–1313. https://doi.org/10.1016/j.procbio.2016.05.021
Dujardin E, Peet C, Stubbs G, Culver JN, Mann S (2003) Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Lett 3:413–417
Lee SY, Royston E, Culver JN, Harris MT (2005) Improved metal cluster deposition on a genetically engineered tobacco mosaic virus template. Nanotechnology 16:435–441. https://doi.org/10.1088/0957-4484/16/7/01
Thangavelu RM, Ganapathy R, Ramasamy P, Krishnan K (2020) Fabrication of virus metal hybrid nanomaterials: an ideal reference for bio semiconductor. Arabian J Chem 13:2750–2765. https://doi.org/10.1016/j.arabjc.2018.07.006
Dahoumane SA, Wujcik EK, Jeffryes C (2016) Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzyme Microb Technol 95:13–27. https://doi.org/10.1016/j.enzmictec.2016.06.008
Sathishkumar RS, Sundaramanickam A, Srinath R, Ramesh T, Saranya K, Meena M, Surya P (2019) Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J Saudi Chem Soc 23:1180–1191. https://doi.org/10.1016/j.jscs.2019.07.008
Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154
Ranoszek-Soliwoda K, Tomaszewska E, Małek K, Celichowski G, Orlowski P, Krzyzowska M, Grobelny J (2019) The synthesis of monodisperse silver nanoparticles with plant extracts. J Colloids Surf B: Biointerfaces 177:19–24. https://doi.org/10.1016/j.colsurfb.2019.01.037
Kandeel M, Akhtar T, Zaheer T, Ahmad S, Ashraf U, Omar M (2022) Anti-parasitic applications of nanoparticles: a review. Pak Vet J 42:135–140. https://doi.org/10.29261/pakvetj/2022.040
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol adv 31:346–356. https://doi.org/10.1016/j.biotechadv.2013.01.003
Naghdi M, Taheran M, Brar SK, Verma M, Surampalli RY, Valéro JR (2015) Green and energy-efficient methods for the production of metallic nanoparticles. Beilstein J Nanotechnol 6:2354–2376. https://doi.org/10.3762/bjnano.6.243
Ueno S, Nakashima K, Sakamoto Y, Wada S (2015) Synthesis of silver-strontium titanate hybrid nanoparticles by sol-gel-hydrothermal method. Nanomaterials 5:386–397. https://doi.org/10.3390/nano5020386
Li X, Kim N, Youn S, An TK, Kim J, Lim S, Kim SH (2019) Sol–gel-processed organic–inorganic hybrid for flexible conductive substrates based on gravure-printed silver nanowires and graphene. Polymers 11:1–14. https://doi.org/10.3390/polym11010158
Muromachi T, Tsujino T, Kamitani K, Maeda K (2006) Application of functional coatings by sol-gel method. J Sol-Gel Sci Technol 40:267–272. https://doi.org/10.1007/s10971-006-8386-7
Milea CA, Bogatu C, Duta A (2011) The influence of parameters in silica sol-gel process. Bull Transilvania Univ Brasov 4:59–66
Noritomi H, Umezawa Y, Miyagawa S, Kato S (2011) Preparation of highly concentrated silver nanoparticles in reverse micelles of sucrose fatty acid esters through solid-liquid extraction method. Adv Chem Eng Sci 1:299–304. https://doi.org/10.4236/aces.2011.14041
Singha D, Barman N, Sahu K (2014) A facile synthesis of high optical quality silver nanoparticles by ascorbic acid reduction in reverse micelles at room temperature. J Colloid Interface Sci 413:37–42. https://doi.org/10.1016/j.jcis.2013.09.009
Piszczek P, Radtke A (2018) Silver nanoparticles fabricated using chemical vapor deposition and atomic layer deposition techniques: properties, applications and perspectives: review. Noble and Precious Metals; Properties, Nanoscale Effects and Applications: InTech 187–213
Zhang KX, Wen X, Yao CB, Li J, Zhang M, Li QH, Wu JD (2018) Synthesis, structural and optical properties of silver nanoparticles uniformly decorated ZnO nanowires. Chem Phys Lett 698:147–151. https://doi.org/10.1016/j.cplett.2018.03.018
Chen C, Wang L, Yu H, Jiang G, Yang Q, Zhou J, Zhang J (2008) Study on the growth mechanism of silver nanorods in the nanowire-seeding polyol process. Mater Chem Phys 107:13–17. https://doi.org/10.1016/j.matchemphys.2007.06.048
Zhang P, Wyman I, Hu J, Lin S, Zhong Z, Tu Y, Wei Y (2017) Silver nanowires: Synthesis technologies, growth mechanism and multifunctional applications. Mater Sci Eng B 223:1–23. https://doi.org/10.1016/j.mseb.2017.05.002
Mohanty US (2011) Electrodeposition: a versatile and inexpensive tool for the synthesis of nanoparticles, nanorods, nanowires, and nanoclusters of metals. J Appl Electrochem 41:257–270. https://doi.org/10.1007/s10800-010-0234-3
Ali A, Zafar H, Zia MI, ulHaq, AR, Phull, JS, Ali, A, Hussain (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 9:49–67. https://doi.org/10.2147/NSA.S99986
Mafuné F, Kohno J-y, Takeda Y, Kondow T (2002) Full physical preparation of size-selected gold nanoparticles in solution: laser ablation and laser-induced size control. J Phys Chem B 106:7575–7577. https://doi.org/10.1021/jp020577y
Bajwa HUR, Khan MK, Abbas Z et al (2022) Nanoparticles: synthesis and their role as potential drug candidates for the treatment of parasitic diseases. Life 12:1–16. https://doi.org/10.3390/life12050750
Cameron P, Gaiser BK, Bhandari B, Bartley PM, Katzer F, Bridle H (2016) Silver nanoparticles decrease the viability of Cryptosporidium parvum oocysts. Appl Environ Microbiol 82:431–437. https://doi.org/10.1128/AEM.02806-15
Saha SK, Roy P, Saini P, Mondal MK, Chowdhury P, Babu SPS (2016) Carbohydrate polymer inspired silver nanoparticles for filaricidal and mosquitocidal activities: a comprehensive view. Carbohydr polym 137:390–401. https://doi.org/10.1016/j.carbpol.2015.11.007
Ullah A, Sun H, Hakim Yang X, Zhang X (2018) A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol Plant 162:439–454. https://doi.org/10.1111/ppl.12651
Li Y, Guo M, Lin Z, Zhao M et al (2016) Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. Int J Nanomed 11:6693–6702. https://doi.org/10.2147/IJN.S122666
Zou X, Li P, Lou J, Zhang H (2017) Surface coating-modulated toxic responses to silver nanoparticles in Wolffia globosa. Aquat Toxicol 189:150–158. https://doi.org/10.1016/j.aquatox.2017.06.010
Gagne RB, Crooks KR, Craft ME et al (2022) Parasites as conservation tools. Conserv Biol 36:1–12. https://doi.org/10.1111/cobi.13719
Gupta R, Xie H (2018) Nanoparticles in daily life: applications, toxicity and regulations. J Environ Pathol 37:209–230. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018026009
Li P, Rios Coronado PE, Longstaff XR, Tarashansky AJ, Wang B (2018) Nanomedicine approaches against parasitic worm infections. Adv healthc mater 7:1–18. https://doi.org/10.1002/adhm.201701494
Sarangi B, Jana U, Sahoo J, Mohanta GP, Manna PK (2018) Systematic approach for the formulation and optimization of atorvastatin loaded solid lipid NANOAPARTICLES using response surface methodology. Biomed Microdevices 20:1–12. https://doi.org/10.1007/s10544-018-0285-5
Parish T (2019) Steps to address anti-microbial drug resistance in today’s drug discovery. Expert Opin Drug Discov 14:91–94. https://doi.org/10.1080/17460441.2019.1550481
Ghafoorianfar S, Ghorani-Azam A, Mohajeri SA, Farzin D (2020) Efficiency of nanoparticles for treatment of ocular infections: systematic literature review. J Drug Deliv Sci Technol 57:1–7. https://doi.org/10.1016/j.jddst.2020.101765
Sun Y, Chen D, Pan Y, Qu W et al (2019) Nanoparticles for antiparasitic drug delivery. Drug Deliv 26:1206–1221. https://doi.org/10.1080/10717544.2019.1692968
Novobilský A, Höglund J (2015) First report of closantel treatment failure against Fasciola hepatica in cattle. Int J Parasitol: Drugs Drug Resist 5:172–177. https://doi.org/10.1016/j.ijpddr.2015.07.003
Rehman A, Ullah R, Uddin I, Zia I, Rehman L, Abidi SM (2019) In vitro anthelmintic effect of biologically synthesized silver nanoparticles on liver amphistome, Gigantocotyle explanatum. Exp Parasitol 198:95–104. https://doi.org/10.1016/j.exppara.2019.02.005
Tomar RS, Preet S (2017) Evaluation of anthelmintic activity of biologically synthesized silver nanoparticles against the gastrointestinal nematode, Haemonchus contortus. J helminthol 91:454–461. https://doi.org/10.1017/S0022149X16000444
Barbosa ACMS, Silva LPC, Ferraz CM, Tobias FL et al (2019) Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int J Nanomed 14:2341–2348. https://doi.org/10.2147/IJN.S193679
Preet S, Tomar RS (2017) Anthelmintic effect of biofabricated silver nanoparticles using Ziziphus jujuba leaf extract on nutritional status of Haemonchus contortus. Small Rumin Res 154:45–51
Venjakob PL, Thiele G, Clausen PH, Nijhof AM (2017) Toxocara vitulorum infection in German beef cattle. Parasitol Res 116:1085–1088. https://doi.org/10.1007/s00436-017-5393-2
Bahaaeldine MA, El Garhy M, Fahmy SR, Mohamed AS (2022) In vitro anti-Toxocara vitulorum effect of silver nanoparticles. J Parasit Dis 46:409–420. https://doi.org/10.1007/s12639-021-01464-0
Rahimi MT, Ahmadpour E, Esboei BR et al (2015) Scolicidal activity of biosynthesized silver nanoparticles against Echinococcus granulosus protoscolices. Int J Surg 19:128–133. https://doi.org/10.1016/j.ijsu.2015.05.043
Salih TA, Hassan KT, Majeed SR, Ibraheem IJ, Hassan OM, Obaid AS (2020) In vitro scolicidal activity of synthesised silver nanoparticles from aqueous plant extract against Echinococcus granulosus. Biotechnol Rep 28:1–6. https://doi.org/10.1016/j.btre.2020.e00545
Rashid MM, Ferdous J, Banik S, Islam MR, Uddin AM, Robel FN (2016) Anthelmintic activity of silver-extract nanoparticles synthesized from the combination of silver nanoparticles and M. charantia fruit extract. BMC Complement Altern Med 242:1–6. https://doi.org/10.1186/s12906-016-1219-5
Sharma G, Kalra SK, Tejan N, Ghoshal U (2020) Nanoparticles based therapeutic efficacy against Acanthamoeba: updates and future prospect. Exp Parasitol 218. https://doi.org/10.1016/j.exppara.2020.108008
Nafari A, Cheraghipour K, Sepahvand M, Shahrokhi G, Gabal E, Mahmoudvand H (2020) Nanoparticles: new agents toward treatment of leishmaniasis. Parasite Epidemiol Control 10:1–10. https://doi.org/10.1016/j.parepi.2020.e00156
Shin SW, Song IH, Um SH (2015) Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 5:1351–1365. https://doi.org/10.3390/nano5031351
Kausar S, Khan W, Dwivedi S, Azam A (2020) Antifilarial effect of nanocomposite of silver nanoparticles with nitazoxanide against the microfilariae of Setaria cervi-infected albino rats. Naunyn-Schmiedeberg’s Arch Pharmacol 393:1341–1356. https://doi.org/10.1007/s00210-020-01821-5
Ilavarashi P, Rani N, Velusamy R, Raja MJ, Ponnudurai G (2019) In-vitro anthelmintic evaluation of synthesized silver nanoparticles of Moringa oleifera seeds against strongyle nematode of small ruminants. J Pharmacogn Phytochem 8:2116–2121
Mirzaei Y, Hamad SM, Barzinjy AA, Faris VM, Karimpour M, Ahmed MH (2022) In vitro effects of the green synthesized silver and nickel oxide nanoparticles on the motility and egg hatching ability of Marshallagia marshalli. Emergent Mater 5:1705–1716. https://doi.org/10.1007/s42247-022-00420-9
Saini P, Saha SK, Roy P, Chowdhury P, Babu SPS (2016) Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp Parasitol 160:39–48. https://doi.org/10.1016/j.exppara.2015.11.004
Jari SD, Yousif JJ (2020) Therapeutic effects of silver nanoparticles loaded with albendazole, mebendazole drugs in male albino mice infected with hydatid cysts. Int Res J Adv Sci 1:13–18
Kaiaty AM, Salib FA, El-Gameel SM, Abdel Massieh ES, Hussien AM, Kamel MS (2023) Emerging alternatives to traditional anthelmintics: the in vitro antiparasitic activity of silver and selenium nanoparticles, and pomegranate (Punica granatum) peel extract against Haemonchus contortus. Trop Anim Health Prod 55:1–14. https://doi.org/10.1007/s11250-023-03722-0
Nassef NE, Saad AGE, Harba NM, Beshay EV, Gouda MA, Shendi SS, Mohamed ASED (2019) Evaluation of the therapeutic efficacy of albendazole-loaded silver nanoparticles against Echinococcus granulosus infection in experimental mice. J Parasit Dis 43:658–671. https://doi.org/10.1007/s12639-019-01145-z
Bilia AR, Piazzini V, Guccione C, Risaliti L, Asprea M, Capecchi G, Bergonzi MC (2017) Improving on nature: the role of nanomedicine in the development of clinical natural drugs. Planta Med 83:366–381. https://doi.org/10.1055/s-0043-102949
Molento MB, Arenal A (2020) The breakthrough of nanotechnology to veterinary parasitology research. LALIOTIS, GP Current research in agriculture and veterinary science London: Publisher Int 1 49 54 https://doi.org/10.9734/bpi/cravs/v1
Gaafar MR, El-Zawawy LA, El-Temsahy MM, Shalaby TI, Hassan AY (2019) Silver nanoparticles as a therapeutic agent in experimental cyclosporiasis. Exp Parasitol 207:1–12. https://doi.org/10.1016/j.exppara.2019.107772
Benelli G, Maggi F, Romano D, Stefanini C, Vaseeharan B, Kumar S, Higuchi A, Alarfaj AA, Mehlhorn H, Canale A (2017) Nanoparticles as effective acaricides against ticks: a review. Ticks Tick-borne Dis 8:821–826. https://doi.org/10.1016/j.ttbdis.2017.08.004
Farooq U, Ahmad T, Khan A et al (2019) Rifampicin conjugated silver nanoparticles: a new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumoniae. Int J Nanomed 14:3983–3993. https://doi.org/10.2147/IJN.S198194
Calderon-Nieva D, Goonewardene KB, Gomis S, Foldvari M (2017) Veterinary vaccine nanotechnology: Pulmonary and nasal delivery in livestock animals. Drug Deliv Transl Res 7:558–570. https://doi.org/10.1007/s13346-017-0400-9
Khezerlou A, Alizadeh-Sani M, Azizi-Lalabadi M, Ehsani A (2018) Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb Pathog 123:505–526. https://doi.org/10.1016/j.micpath.2018.08.008
Neethirajan S, Tuteja SK, Huang S-T, Kelton D (2017) Recent advancement in biosensors technology for animal and livestock health management. Biosens Bioelectron 98:398–407. https://doi.org/10.1016/j.bios.2017.07.015
El-Kosary S, Abd Allatif AM, Stino RG, Hassan MM, Kinawy AA (2020) Effect of silver nanoparticles on micropropagation of date palm (Phoenix dactylifera L., Cv. Sewi and Medjool). Plant Arch 20:9701–9706
Burange PJ, Tawar MG, Bairagi RA et al (2021) Synthesis of silver nanoparticles by using Aloe vera and Thuja orientalis leaves extract and their biological activity: a comprehensive review. Bull Natl Res Cent 45:1–13. https://doi.org/10.1186/s42269-021-00639-2
Verma P, Maheshwari SK (2019) Applications of Silver nanoparticles in diverse sectors. Int J Nano Dimens 10:18–36
Krug HF (2014) Nanosafety research—are we on the right track? Angewandte Chemie Int Ed 53:12304–12319. https://doi.org/10.1002/anie.201403367
He X, Hwang HM (2016) Nanotechnology in food science: functionality, applicability, and safety assessment. J Food Drug Anal 24:671–681. https://doi.org/10.1016/j.jfda.2016.06.001
McShan D, Ray PC, Yu H (2014) Molecular toxicity mechanism of nanosilver. J Food Drug anal 22:116–127. https://doi.org/10.1016/j.jfda.2014.01.010
Alghuthaymi MA, Almoammar H, Rai M, Said-Galiev E, Abd-Elsalam KA (2015) Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol Biotechnol Equip 29:221–236. https://doi.org/10.1080/13102818.2015.1008194
Buzea C, Pacheco I, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:MR17–MR71. https://doi.org/10.1116/1.2815690
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1:325–327. https://doi.org/10.1002/smll.200400093
Schubert D, Dargusch R, Raitano J, Chan SW (2006) Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun 342:86–91. https://doi.org/10.1016/j.bbrc.2006.01.129
Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem 15:897–900. https://doi.org/10.1021/bc049951
Bosi S, Da Ros T, Spalluto G, Prato M (2003) Fullerene derivatives: an attractive tool for biological applications. Eur J Med Chem 38:913–923. https://doi.org/10.1016/j.ejmech.2003.09.005
Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, Brar SK (2017) Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng 2:1–21. https://doi.org/10.1007/s41204-017-0029-4
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275:496–502. https://doi.org/10.1016/j.jcis.2004.03.003
El-Shanshoury AE-RR, ElSilk SE, Ebeid ME (2011) Extracellular biosynthesis of silver nanoparticles using Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, and Streptococcus thermophilus ESh1 and their antimicrobial activities. ISRN Nanotechnol 2011:1–7. https://doi.org/10.5402/2011/385480
Velmurugan P, Cho M, Lim SS, Seo SK, Myung H, Bang KS, Sivakumar S, Cho KM, Oh BT (2015) Phytosynthesis of silver nanoparticles by Prunus yedoensis leaf extract and their antimicrobial activity. Mater Lett 138:272–275. https://doi.org/10.1016/j.matlet.2014.09.136
Banerjee P, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess 1:1–10. https://doi.org/10.1186/s40643-014-0003-y
Acknowledgements
The authors extend their appreciation to the Deanship of Scientifc Research at King Khalid University for funding this work through Large Groups Project under grant number (RGP. 2/11/44).
Author information
Authors and Affiliations
Contributions
SM, LMA, MZA, MM, WQ, AAAD, and RZA contributed equally in conceptualization, data analysis, manuscript writing (original draft), and review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mustafa, S., Alharbi, L.M., Abdelraheem, M.Z. et al. Role of Silver Nanoparticles for the Control of Anthelmintic Resistance in Small and Large Ruminants. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04132-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04132-5