Abstract
Background
Gastrointestinal nematode (GIN) infections are common in domestic sheep and impact directly and indirectly on the health of infected animals as well as on the associated economic production. In this study, we aim at summarizing the current knowledge on the influence of GIN infections on sheep production by conducting a systematic review. A subsequent meta-analysis of relevant studies was performed to provide an estimate of the effect of GIN infections on weight gain, wool production and milk yield.
Methods
A literature search was performed on the CAB, Pubmed and Web of Science database for the period 1960–2012. Inclusion criteria were: 1) Measurement of at least one production parameter. 2) Comparison between groups of sheep with different nematode burdens. 3) Same conditions regarding all aspects except parasite burden between groups. 4) Quantitative measurements of one or more production traits.
Results
Altogether, 88 studies describing 218 trials were included in this review. The majority of studies (86 %) reported that GIN infections had a negative effect on production but this was reported to be statistically significant in only 43 % of the studies. Meta-analysis indicated that performances of sheep infected with nematodes was 85, 90 and 78 % of the performance in uninfected individuals for weight gain, wool production and milk yield respectively. Our results suggest a possible reporting bias or small study effect for the estimation of the impact of GIN infections on weight gain. Finally, a general linear model provided an estimate for the decrease in weight gain in relation to the increase in faecal egg count of nematodes.
Conclusion
This study underlines the importance of GIN infections for sheep production and highlights the need to improve parasite management in sheep, in particular in face of challenges such as anthelmintic resistance.
Similar content being viewed by others
Background
Gastro-intestinal parasitism is one of the most common infections in livestock. Clinical signs and sequelae are dependent on the parasite fauna present and the intensity of infection. In sheep, these can range from subclinical weight loss to lethal pathologies such as anaemia, diarrhoea and severe protein loss [1]. In addition, parasitism can have indirect consequences on metabolism such as mobilisation of proteins for an immune-response, reduced feed intake due to anorexia or increased susceptibility to other pathogens [2–4]. Since the 1960s the use of anthelmintics has become an important strategy to control nematode infections in livestock and increase their production performance [5]. For example, Sanchez et al. [6] reported the results of a meta-analysis which concluded that dairy cattle gained an estimated increase in milk production of 0.35 kg/day following treatment against gastro-intestinal nematodes.
According to the Food and Agriculture Organisation [7] the sheep population amounted to 1.2 billion in 2012, distributed as follow: Asia, 44.9, Africa, 27.6, Europe, 11.1, Oceania, 9.1 and Americas, 7.3 %. Worldwide, sheep production for 2012 was 10 million tons of milk, 8 million tons of meat and 2 million tons of wool. Distribution of meat production is correlated with distribution of sheep population whereas milk production is mainly based in the Mediterranean region and the Near East and wool production is proportionally more important in Oceania and Asia [7, 8].
Sheep represent an important source of income in many countries [8, 9] and although the effects of parasitism on production have been recognized [10], there is still a need to quantify these losses. Anthelmintic resistance and climate change is likely to alter the geographical distribution of parasites and their impact on production animals, thus increasing the need for a clear understanding of the cost of parasitism in order to develop sustainable control strategies [10, 11].
Systematic reviews and meta-analysis have been widely used to summarize results of different studies made on one particular subject. The increased sample size obtained when combining studies as well as the possibility to identify error sources such as publication bias improve the quality of the analysis and strengthen its conclusions. In particular, in medical research, those methods are frequently used to measure the efficacy of a treatment or assess the relationship between risk factors and a medical condition [12].
Here we undertake a systematic review to identify studies which evaluated the impact of gastrointestinal nematodes on different aspects of sheep production and summarize their results. Meta-analysis was then applied to the data in suitable studies to evaluate the effect of gastrointestinal nematode (GIN) infections in sheep on weight gain, wool production and milk yield, which are the main economic purposes of sheep breeding [9, 13]. Finally, since effects of parasitism are expected to depend on the parasite burden [10], we also analysed the relation between quantitative egg excretion (used as a proxy for parasite burden in young animals [14]) and production performance.
Methods
The methodology followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA, [15]) recommendations for improving the standards of meta-analyses. A PRISMA check list is provided as supplementary material to this publication (see Additional file 1). Statistical analysis and figures were made using the R statistical program [16].
Search strategy
The databases CAB, Pubmed and Web of Science were searched for the period 1960–2012 in order to retrieve relevant studies. Three production traits were taken in consideration: weight gain, milk yield and wool production. Searches were performed using different key words distributed among three search terms: [nematode/parasite/anthelmintic/parasite control] AND [weight/growth/wool/fleece/milk/production] AND [sheep]. All possible combinations of the three terms were used (e.g., anthelmintic AND fleece AND sheep).
Inclusion and exclusion criteria
Studies were first screened by scanning the title and abstract. Suitable studies were retained for more detailed examination. Studies were then selected for inclusion if they met the following criteria:
-
(1).
A production parameter was measured (weight gain in lambs, wool production or milk yield).
-
(2).
There were at least two groups of sheep which differed in their gastro-intestinal nematode burden (e.g., infected sheep group vs control or dewormed group vs control).
-
(3).
There were no other reported differences between the groups (e.g., feeding, breed, housing, age, infection with trematodes).
-
(4).
The report quantified the production of each group or whether there was a significant difference between groups.
For studies describing more than one trial, each trial was included separately in the review. Additionally, for studies where more than one group were compared to the control group, each group being compared with the control group was considered as a separate trial. Finally, for studies measuring more than one production trait, the recorded gain in each production trait was considered as a separate trial.
Trials were classified into two categories:
-
a)
Infection/control trials: trials with an infected group (INF) and a control group (CONT) with no or a negligible nematode infection (animals raised and kept in a nematode-free environment or regularly treated and with a mean faecal egg count (FEC) < 50 eggs per gram (EPG) determined by repeated measurements over the trial’s duration).
-
b)
Burden trial: Trials which compared production between two groups of nematode infected sheep but in which one group had a high parasite burden (HPAR) and the other group had a lower burden (LPAR).
Subsequently, only trials of the type infection/control were included in a meta-analysis of the effect of infection status on performance. In addition an analysis on the effect of nematode burden on performance was undertaken using all trials (infection/control and burden types) for which FEC was monitored in every group (based on repeated measurements over the duration of the trial).
Effect of infection status on performance
Using the meta and metafor packages in R [17, 18], a meta-analysis was undertaken to evaluate the effect of infection status on production. To construct a confidence interval around the final gain in production, only trials reporting a standard error of the measured outcome were included in the analysis.
A standardized measurement of the gain in production was obtained by computing the ratio of the performance in the INF group over the performance in the control group (no parasite burden). This allowed comparison between different studies, since the reported performance (in grams of body weight/fleece or litres milk) can be influenced by other factors such as breed, feeding or trial duration or was measured with different units between different studies (e.g., wool production measured either in grams of wool at shearing or in mm wool growth).
Since this standardized measurement is a ratio, logarithmic transformation, as described in [19], was used for the computation of confidence intervals and to perform further analysis.
Analysis was performed separately for the three production traits (weight gain, wool production and milk yield) as well as for the type of nematode infection: either mixed species infection or mono-infection with Haemonchus contortus, Trichostrongylus colubriformis or Teladorsagia (Ostertagia) circumcincta. Additionally, only studies performed on growing animals less than one year old were included in analysis measuring weight gain.
Linear regression test for funnel plot asymmetry [20] was conducted to control for publication bias or small-study effect and the fill-and-trim method [21] was used to compute an adjusted estimate of the overall effect when needed.
Relation between egg excretion and performance
We built a generalized linear model (GLM) to estimate the impact on production in relation to the faecal nematode egg output. The measured outcome was defined as the log-transformed ratio of production of the infected group over the control. In addition to the log-transformed difference in mean FEC between the groups five additional explanatory variables were included in the model: 1) the absolute value of the latitude at which the trial was conducted (ranging from 0 at the equator to 90 at each pole) which served as proxy for a possible effect of climate [22, 23]; 2) trial duration in weeks, since the impact of a pathogen might not only depend on infection intensity, but also on infection duration [24] or development of immunity by the host [2]; 3) age classes of the animals (1–6 months or 7–12 months) since effect of parasitism and host response can vary with the age of the lambs [25, 26];4) study design (infected vs control, treated vs untreated or other) was added as a predictive variable since infection pressure and its fluctuation over the trial duration might differ between the different type of trials. In addition, infection course and host response might differ between experimentally or naturally acquired parasite infection [27]; 5) FEC diagnostic method (gravitational or centrifugal) was also included since it might influence the estimate of parasite burden in animals [28, 29]. Additionally, trials were assigned weight in the model according to their sample size. The model was constructed using backward selection based on the Aikake Informaton Criterion (AIC).
Similarly to the meta-analysis on the effect of infection status, we considered trials separately, depending on the three production traits measured as well as on the species of nematodes infecting the animals. However only trials measuring weight gain in lambs with mixed parasite infection were in sufficient quantity to provide a robust model (n = 73) and thus, only those trials were used for modelling. Finally, we also investigated the relationship between FEC and nematode burden in studies which necropsied animals and performed a worm count of the whole gastrointestinal tract.
Results
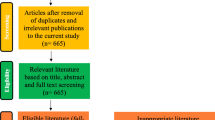
Searching the three databases, a total of 45,402 results corresponding to 11,873 studies were obtained. Of these, 265 studies remained after an initial screening of titles and abstracts. Finally 85 studies were included following full paper review. The main reasons for excluding studies were: study on agent other than nematodes, study on species other than sheep, production parameters of interest not measured and difference between the experimental groups regarding aspects other than parasite burden (e.g., food, breed). During this process, three additional studies were identified from the cited references of screened studies and also included in the review resulting in a total of 88 studies [30–117].
These 88 studies described a total of 218 trials. Twenty-two studies described only one trial. The other 66 studies included at least two trials. Mean sample size in the trials was 49 (median: 20, range: 8–500) and average trial duration was 16 weeks. Gain in production was assessed by treating animals with anthelmintics in 42 studies, through experimental infection in 40 studies, through different pasture management methods (e.g., pasture rotation) in five studies and by comparing animals with naturally high and low FEC in one study. Studies originated from 23 different countries. The United-Kingdom and Australia were the countries with the most studies (18 and 12, respectively) and account for more than one third of the total studies included in this review (Table 1).
Table 2 shows a summary of the reported effect of parasitism on production in sheep. Altogether, 187 trials (85.8 %) reported a negative effect of nematode infection on production, with 94 (43.1 %) of them reporting a statistically significant effect. In contrast, a positive effect of parasitism on production was found in 24 trials (10.9 %) and seven (3.2 %) trials reported no differences in production between parasitised and control animals.
Altogether, statistical testing of the effect of parasitism on production was reported in 183/218 trials. There was no significant difference in the proportion of trials reporting a p-value between trials describing a negative effect of parasitism and those reporting a positive effect (159/187 vs 18/24, Fisher exact test: p = 0.237).
However, a larger proportion of trials reported a significant negative effect of parasitism compared to trials reporting a significant positive effect (94/159 vs 2/18, Fisher exact test: p < 0.001).
Effect of infection status on performance
A total of 94 trials were of the type infection/control and met requirements to be included in the meta-analysis (70 trials measuring weight gain, 5 trials measuring milk yield and 19 trials measuring wool production).
In 78/94 trials, a negative effect of parasitism on production was reported (weight gain: 59/70, milk yield: 5/5, wool production: 14/19). However, in 14 trials (weight gain: 10/70, wool production: 4/19) parasitism was associated with an increased performance. Finally, in two trials (one measuring weight gain and one measuring wool production), the authors reported there were no differences between infected and control animals.
Results of the meta-analysis are summarized in Fig. 1 and Table 3. Test for funnel plot asymmetry indicated a possible bias for trials reporting weight gain (p = 0.032) but not for wool production (p = 0.307) and milk yield (p = 0.336). Fig. 2 shows the funnel plots for the three production traits.
Forest plots of 94 trials included in the meta-analysis of impact of gastro-intestinal nematode infection on weight gain (a, n = 70), wool production (b, n = 19) and milk yield (c, n = 5) in sheep. Black dots represent the log-transformed ratio of performance of the infected over the control group in each trial. Dot sizes are proportional to the sample sizes in the trial and horizontal bars give the standard error of the estimate. Vertical dotted lines indicate the zero (no effect of nematode infection on production) and vertical continuous lines show the overall estimate for all the trials in each performance trait
Funnel plots with 95 % pseudo-confidence limits of 94 trials included in the meta-analysis of impact of nematodes on weight gain (a, n = 70), wool production (b, n = 19) and milk yield (c, n = 5) in sheep. Treatment effect (log-transformed ratio of performance of infected over control animals) is given on the X-axis and standard error of the estimate is represented on the Y-axis
Altogether, estimates for the production ratio of infected animals over control were:
-
0.77 (95 % CI: 0.74–0.79) for weight gain or 0.85 (95 % CI: 0.82–0.88) after adjustment for reporting bias,
-
0.90 (95 % CI: 0.86–0.93) for wool production,
-
0.78 (95 % CI: 0.73–0.84) for milk yield.
In 75 trials, mean FEC over trial duration were reported for the infected group and ranged from 100 to 12,000 EPG (for details see table 3).
Relation between parasite excretion and performance
The best model (AIC: 27.532) included only increases in FEC as a predictor of the weight gain ratio between HPAR and LPAR groups (21.37 % of deviance explained). Fig. 3 shows the observed effect of parasitism recorded in the trials and the estimate of the model.
Decrease in weight gain of sheep by increasing infection level with mixed species of gastrointestinal nematodes. Mean difference in faecal egg counts between low parasite burden animals (LPAR) and high parasite burden animals (HPAR) is used as an indicator of level of infection and shown on the X-axis. Y-axis shows the ratio of weight gain of HPAR over LPAR. The continuous line shows the estimated effect of nematode infection with a 95 % confidence interval (dotted lines) computed with a Generalized Linear Model using the results of 73 trials (black dots with sizes proportional to sample size of the trials)
Altogether, by mixed species infection, an increase in FEC of 100, 1’000 and 10’000 EPG resulted in the HPAR lambs gaining 0.85, 0.71 and 0.6 times the weight of the LPAR lambs, respectively).
Finally, in 9 studies, lambs from either the HPAR groups or both HPAR and LPAR groups were necropsied and worm counts of the whole gastrointestinal tracts were performed. Altogether, worm count ranged from 30 to 41’718 and there was a positive correlation between mean FEC before slaughter and worm count (n = 26, spearman’s rho = 0.71, p < 0.001).
Discussion
In this systematic review, a number of studies describing the relation between parasite infection and production in sheep were identified. The large majority of studies focused on the effect of parasitism on weight gain and relatively few studies measured other parameters such as wool production or milk yield.
Altogether, although the large majority of the trials reported a negative effect of parasitism on production, only 58.3 % of the trials for which a p-value was provided found this effect to be statistically significant. This lack of statistical significance could be due to the relatively small sample size in many of the studies as the median sample size in all the studies included in this review was only 20.
When looking at the trials comparing parasite-free and infected animals, the results of the meta-analysis indicate that, in parasite infected animals, the production in terms of weight gain, wool, and milk is respectively 77, 90 and 78 % of the production of parasite-free animals. Analysing the separate impact of different species of nematodes gave similar estimates, with wool production being less influenced than weight gain by parasitism.
Testing for funnel plot asymmetry indicated that trials measuring weight gain were probably biased. Therefore the adjusted estimate of infected animals gaining 85 % of the weight of non-infected animals seems more reliable than the 77 % unadjusted estimate.
In contrast, no bias was detected following the meta-analyses of trials measuring wool production and milk yield. However, testing for bias is unreliable when the meta-analysis includes a small number of studies [118]. Thus there is the possibility of bias in the estimates of the effect of parasitism on wool production and milk yield presented in this review. If that is the case, it is likely that, similarly to weight gain, our analysis overestimates the true impact of parasitism on those production traits.
Nevertheless, our results indicate that milk yield and weight gain are much more influenced by parasitism than wool production. Coop et al. [2] proposed that sheep respond to parasitism by shifting resource allocation with higher priority to maintaining vital body function, with other function such as weight gain and lactation being given a lower priority, and thus more likely to receive less resources in case of parasitism. It is possible that wool growth is part of sheep vital functions, which might explain the smaller effect of parasitism on this parameter.
In a review of the effect of parasitism in dairy cow production, Sanchez et al. [6] noted that level of parasitic infection is likely to be an important factor determining the effect on the milk yield and probably accountable for the large variation of the effect reported in the different studies. Similarly, only a minority of the studies included in the present review reported a level of infection, either by describing the initial parasite dose in case of experimental infection trials or by sacrificing animals to perform a post-mortem worm count.
In another meta-analysis, Kipper et al. [119] estimated that parasite-infected pigs had a daily weight gain 31 % inferior than non-infected individuals. Kipper did not discriminate between the different species of parasite when estimating their impact. He argued that the main effect of parasitism was due to the host adaptation to an infection and its immune response rather than to the species involved. The present study seems to support this argument since the estimate of the impact of the different nematode species considered separately were quite similar to the overall estimates for each production trait. However, because of the small number of trials for each separate nematode species, those estimates have to be interpreted with caution.
While FEC is usually considered a reliable indicator of nematode burden in small ruminants [14, 72, 120], some authors pointed out that the relationship between both variables might be more complex and involves other factors such as parasite density and diversity [121, 122] or host age and development of immunity [123, 124]. In this review, we found a strong relationship between FEC at slaughter and gastrointestinal worm count in lambs. It must be noted, though that those were averaged values which did not allow to account for individual variability and that the amount of groups for which worm count was reported was small (n = 26).
In the GLM presented here, increase in FEC was the only variable included in the best model. It was significantly associated with a decrease in weight gain and explained 21 % of the total deviance. None of the other variables tested in the analysis were selected in the final model. However, because of a strong heterogeneity and a lack of precise information in the included studies, we summarized the variables study design and FEC diagnostic method into two or three rough categories (e.g., gravitational vs centrifugal). This simplification might limit the ability of the model to detect an effect for those variables. For the same reason, other potentially relevant predictors such as breed, diet or co-infection with other pathogens could not be included in the analysis.
Although, alternative indicator such as plasma antibodies or pepsinogen level have been proposed [125], the results of this review corroborate that FEC can help evaluate nematode burden and its impact on weight gain in lambs. Additionally, procedures requiring blood sampling of individuals is more expensive in term of time and resources than FEC which make them less attractive for monitoring purpose. However, other less invasive parameters such as body condition or FAMACHA scores have proven themselves helpful in the frame of targeted selective treatments [126] and should be further propagated.
Finally, most of the studies identified with naturally infected animals used classical anthelmintic compounds in their experimental design. Although the efficacy of such products is widely acknowledged, increasing resistance of GIN to anthelmintics is reported worldwide [127, 128]. This review demonstrates that an increase in non-responsiveness to classical anthelmintics will have an important impact on sheep production and underlines the need for alternatives to chemical worm control such as pasture management, resistant breed or vaccination [129].
Conclusion
This study confirms the importance of GIN infections on sheep performance and underlines the advantages of parasite control in production animals. The consequences of GIN infections seem to be similar for different species of parasites but seem to influence milk yield and weight gain more than wool production.
References
Pugh DG, Baird N. Sheep & goat medicine. USA: Elsevier Health Sciences; 2012.
Coop RL, Kyriazakis I. Nutrition–parasite interaction. Vet Parasitol. 1999;84:187–204.
Moreau E, Chauvin A, Moreau E, Chauvin A. Immunity against Helminths: Interactions with the Host and the Intercurrent Infections. BioMed Res Int BioMed Res Int. 2010.
Sykes AR, Coop RL. Interaction between nutrition and gastrointestinal parasitism in sheep. N Z Vet J. 2001;49:222–6.
Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–81.
Sanchez J, Dohoo I, Carrier J, DesCôteaux L. A meta-analysis of the milk-production response after anthelmintic treatment in naturally infected adult dairy cows. Prev Vet Med. 2004;63:237–56.
FAO, FAOSTAT Online Statistical Service (Live Animal and Livestock Primary datasets).2013, http://faostat.fao.org/site/569/DesktopDefault.aspx?PageID=569#ancor. Accessed 03 Sep 2015.
Zygoyiannis D. Sheep production in the world and in Greece. Small Rumin Res. 2006;62:143–7.
Morris ST. Economics of sheep production. Small Rumin Res. 2009;86:59–62.
Charlier J, van der Voort M, Kenyon F, Skuce P, Vercruysse J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014;30:361–7.
Miller CM, Waghorn TS, Leathwick DM, Candy PM, Oliver A-MB, Watson TG. The production cost of anthelmintic resistance in lambs. Vet Parasitol. 2012;186:376–81.
Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14 Suppl 1:29–37.
Boutonnet J-P. Perspectives of the sheep meat world market on future production systems and trends. Small Rumin Res. 1999;34:189–95.
Cabaret J, Gasnier N, Jacquiet P. Faecal egg counts are representative of digestive-tract strongyle worm burdens in sheep and goats. Parasite Paris Fr. 1998;5:137–42.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA StatementThe PRISMA statement. Ann Intern Med. 2009;151:264–9.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013.
Schwarzer G. Meta: an R package for meta-analysis. 2007.
Viechtbauer W. Metafor: meta-analysis package for R. 2010.
Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–6.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Morgan ER, van Dijk J. Climate and the epidemiology of gastrointestinal nematode infections of sheep in Europe. Vet Parasitol. 2012;189:8–14 [Special Issue: Update on Parasitic Diseases of Sheep].
Rinaldi L, Catelan D, Musella V, Cecconi L, Hertzberg H, Torgerson PR, et al. Haemonchus contortus: spatial risk distribution for infection in sheep in Europe. Geospatial Health. 2015;9:325–31.
Klei TR, McVay CS, Dennis VA, Coleman SU, Enright FM, Casey HW. Effects of duration of infection and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus). Exp Parasitol. 1990;71:393–405.
Smith WD, Jackson F, Jackson E, Williams J. Age immunity to Ostertagia circumcincta: Comparison of the local immune responses of 4 1/2- and 10-month-old lambs. J Comp Pathol. 1985;95:235–45.
Stear MJ, Mitchell S, Strain S, Bishop SC, McKellar QA. The influence of age on the variation among sheep in susceptibility to natural nematode infection. Vet Parasitol. 2000;89:31–6.
Boes J, Medley GF, Eriksen L, Roepstorff A, Nansen P. Distribution of Ascaris suum in experimentally and naturally infected pigs and comparison with Ascaris lumbricoides infections in humans. Parasitology. 1998;117:589–96.
Ballweber LR, Beugnet F, Marchiondo AA, Payne PA. American Association of Veterinary Parasitologists’ review of veterinary fecal flotation methods and factors influencing their accuracy and use—Is there really one best technique? Vet Parasitol. 2014;204:73–80 [SpeciaI Issue: Antiparasitic Drug Use and Resistance in Cattle, Small Ruminants and Equines in the United States - Current Status and Global Perspectives].
Cebra CK, Stang BV. Comparison of methods to detect gastrointestinal parasites in llamas and alpacas. J Am Vet Med Assoc. 2008;232:733–41.
Alba-Hurtado F, Romero-Escobedo E, Muñoz-Guzmán MA, Torres-Hernández G, Becerril-Pérez CM. Comparison of parasitological and productive traits of Criollo lambs native to the central Mexican Plateau and Suffolk lambs experimentally infected with Haemonchus contortus. Vet Parasitol. 2010;172:277–82.
Altaif KI. Effect of anthelmintic treatment on the performance of awassi sheep in Iraq. Trop Anim Health Prod. 1979;11:241–5.
Angulo-Cubillán FJ, García-Coiradas L, Alunda JM, Cuquerella M, de la Fuente C. Biological characterization and pathogenicity of three Haemonchus contortus isolates in primary infections in lambs. Vet Parasitol. 2010;171:99–105.
Arsenos G, Fortomaris P, Papadopoulos E, Kufidis D, Stamataris C, Zygoyiannis D. Meat quality of lambs of indigenous dairy Greek breeds as influenced by dietary protein and gastrointestinal nematode challenge. Meat Sci. 2007;76:779–86.
Athanasiadou S, Kyriazakis I, Jackson F, Coop RL. Consequences of long-term feeding with condensed tannins on sheep parasitised with Trichostrongylus colubriformis. Int J Parasitol. 2000;30:1025–33.
Athanasiadou S, Gray D, Younie D, Tzamaloukas O, Jackson F, Kyriazakis I. The use of chicory for parasite control in organic ewes and their lambs. Parasitology. 2007;134:299–307.
Aumont G, Gruner L, Hostache G. Comparison of the resistance to sympatric and allopatric isolates of Haemonchus contortus of Black Belly sheep in Guadeloupe (FWI) and of INRA 401 sheep in France. Vet Parasitol. 2003;116:139–50.
Bailey JN, Walkden-Brown SW, Kahn LP. Comparison of strategies to provide lambing paddocks of low gastro-intestinal nematode infectivity in a summer rainfall region of Australia. Vet Parasitol. 2009;161:218–31.
Barger IA, Cox HW. Wool production of sheep chronically infected with Haemonchus contortus. Vet Parasitol. 1984;15:169–75.
Beraya, Copeman DB. Seasonal differences in the effect of nematode parasitism on weight gain of sheep and goats in Cigudeg, West Java. J Ilmu Ternak Dan Vet. 1996;2:66–72.
Boa ME, Thamsborg SM, Kassuku AA, Bøgh HO. Comparison of worm control strategies in grazing sheep in Denmark. Acta Vet Scand. 2001;42:57.
Boag B, Thomas RJ. Epidemiological studies on gastro-intestinal nematode parasites of sheep: The control of infection in lambs on clean pasture. Res Vet Sci. 1973.
Bonanno A, Di Miceli G, Di Grigoli A, Frenda AS, Tornambè G, Giambalvo D, et al. Effects of feeding green forage of sulla (Hedysarum coronarium L.) on lamb growth and carcass and meat quality. Animal. 2011;5:148–54.
Bricarello PA, Gennari SM, Oliveira-Sequeira TCG, Vaz CMSL, de Goncalves IG, Echevarria FAM. Response of corriedale and crioula lanada sheep to artificial primary infection with haemonchus contortus. Vet Res Commun. 2002;26:447–57.
Bricarello PA, Amarante AFT, Rocha RA, Cabral Filho SL, Huntley JF, Houdijk JGM, et al. Influence of dietary protein supply on resistance to experimental infections with Haemonchus contortus in Ile de France and Santa Ines lambs. Vet Parasitol. 2005;134:99–109.
Brunsdon RV. The effect of infestation by nematodes of the family trichostrongylidae and the tapeworm, Moniezia expansa, upon the liveweight gain and wool production of young sheep. N Z Vet J. 1964;12:129–34.
Burke JM, Miller JE, Terrill TH. Impact of rotational grazing on management of gastrointestinal nematodes in weaned lambs. Vet Parasitol. 2009;163:67–72.
Butter NL, Dawson JM, Wakelin D, Buttery PJ. Effect of dietary tannin and protein concentration on nematode infection (Trichostrongylus colubriformis) in lambs. J Agric Sci. 2000;134:89–99.
Cardia DFF, Rocha-Oliveira RA, Tsunemi MH, Amarante AFT. Immune response and performance of growing Santa Ines lambs to artificial Trichostrongylus colubriformis infections. Vet Parasitol. 2011;182:248–58.
Carmichael IH. Influences on internal parasitism of sheep in south-east Australia: Studies of (1) internal parasite control on pivot irrigation systems and (2) cobalt nutrition and internal parasite interactions. Wool Technol Sheep Breed. 2002;50.
Coop RL, Sykes AR, Angus KW. The effect of three levels of intake of Ostertagia Circumcincta Larvae on growth rate, food intake and body composition of growing lambs. J Agric Sci. 1982;98:247–55.
Coop R, Graham R, Jackson F, Wright S, Angus K. Effect of experimental Ostertagia circumcincta infection on the performance of grazing lambs. Res Vet Sci. 1985;38:282–7.
Coop R, Smith W, Angus K, Graham R, Wright S, Jackson F. Effect of Ostertagia ostertagi on lamb performance and cross resistance to O circumcincta. Res Vet Sci. 1985;39:200–6.
Coop R, Field A, Graham R, Angus K, Jackson F. Effect of concurrent infection with Ostertagia circumcincta and Trichostrongylus vitrinus on the performance of lambs. Res Vet Sci. 1986;40:241–5.
Coop RL, Huntley JF, Smith WD. Effect of dietary protein supplementation on the development of immunity to Ostertagia circumcincta in growing lambs. Res Vet Sci. 1995;59:24–9.
Costa CTC, Bevilaqua CML, Maciel MV, Camurça-Vasconcelos ALF, Morais SM, Monteiro MVB, et al. Anthelmintic activity of Azadirachta indica A. Juss against sheep gastrointestinal nematodes. Vet Parasitol. 2006;137:306–10.
Cringoli G, Veneziano V, Jackson F, Vercruysse J, Greer AW, Fedele V, et al. Effects of strategic anthelmintic treatments on the milk production of dairy sheep naturally infected by gastrointestinal strongyles. Vet Parasitol. 2008;156:340–5.
Cringoli G, Rinaldi L, Veneziano V, Mezzino L, Vercruysse J, Jackson F. Evaluation of targeted selective treatments in sheep in Italy: Effects on faecal worm egg count and milk production in four case studies. Vet Parasitol. 2009;164:36–43.
Cruz-Rojo MA, Martínez-Valladares M, Álvarez-Sánchez MA, Rojo-Vázquez FA. Effect of infection with Teladorsagia circumcincta on milk production and composition in Assaf dairy sheep. Vet Parasitol. 2012;185:194–200.
Darvill FM, Arundel JH, Brown PB. The Effect of Anthelmintic Treatment of Maiden Ewes in the Periparturient Period on Pasture Contamination and Production of Prime Lambs. Aust Vet J. 1978;54:575–84.
Deligiannis K, Lainas T, Arsenos G, Papadopoulos E, Fortomaris P, Kufidis D, et al. The effect of feeding clinoptilolite on food intake and performance of growing lambs infected or not with gastrointestinal nematodes. Livest Prod Sci. 2005;96:195–203.
Diaz Lira CM, Barry TN, Pomroy WE, McWilliam EL, Lopez-Villalobos N. Willow (Salix spp.) fodder blocks for growth and sustainable management of internal parasites in grazing lambs. Anim Feed Sci Technol. 2008;141:61–81.
Douch PGC, Green RS, Risdon PL. Antibody responses of sheep to challenge with Trichostrongylus colubriformis and the effect of dexamethasone treatmen. Int J Parasitol. 1994;24:921–8.
Dynes RA, Moss RA, Bray AR, McAnulty RW. Effect of weaning age on growth rates of lambs infected by gastrointestinal parasites, 68th New Zealand grassland association conference. 2006.
Fthenakis GC, Papadopoulos E, Himonas C. Effects of three anthelmintic regimes on milk yield of ewes and growth of lambs. J Vet Med Ser A. 2005;52:78–82.
Gibson TE, Everett G. Effect of different levels of intake of Ostertagia circumcingta larvae on the faecal egg counts and weight gain of lambs. J Comp Pathol. 1976;86:269–74.
Githiori JB, Höglund J, Waller PJ, Baker RL. Anthelmintic activity of preparations derived from Myrsine africana and Rapanea melanophloeos against the nematode parasite, Haemonchus contortus, of sheep. J Ethnopharmacol. 2002;80:187–91.
Gómez-Rincón C, Uriarte J, Valderrábano J. Efficiency of Duddingtonia flagrans against Trichostrongyle infections of sheep on mountain pastures. Vet Parasitol. 2006;141:84–90.
Good B, Grennan EJ, Crowley BA, Hanrahan JP. Effect of grazing system and anthelmintic treatment of ewes on parasite challenge and lamb growth. Teagasc: Sheep Research Centre; 2001. p. 38.
Haile A, Tembely S, Anindo DO, Mukasa-Mugerwa E, Rege JEO, Yami A, et al. Effects of breed and dietary protein supplementation on the responses to gastrointestinal nematode infections in Ethiopian sheep. Small Rumin Res. 2002;44:247–61.
Hertzberg H, Meyer A, Kohler L, Falconi F, Ochs H. Einfluss einer einmaligen Injektionsbehandlung mit Doramectin auf den Befall mit gastrointestinalen Nematoden bei gealpten Schafen. Schweizer Arch Tierheilkd. 2001;143:305–11.
Huisden CM, Adesogan AT, Gaskin JM, Courtney CH, Raji AM, Kang T. Effect of feeding Mucuna pruriens on helminth parasite infestation in lambs. J Ethnopharmacol. 2010;127:669–73.
Idika I, Chiejina S, Mhomga L, Nnadi P, Ngongeh L. Correlates of resistance to gastrointestinal nematode infection in Nigerian West African dwarf sheep. Asian Pac J Trop Med. 2012;5:529–32.
Israf DA, Zainal MJ, Ben-Gheshir MA, Rasedee A, Sani RA, Noordin MM. Dietary protein influences on regulation of haemonchus contortus populations in dorsimal lambs. J Helminthol. 1998;72:143–6.
Jacobson C, Pluske J, Besier RB, Bell K, Pethick D. Associations between nematode larval challenge and gastrointestinal tract size that affect carcass productivity in sheep. Vet Parasitol. 2009;161:248–54.
Johnstone I, Coote B, Smart K. Effects of parasite control in the peri-parturient period on lamb birth weight and liveweight gain. Aust J Exp Agric. 1979;19:414–8.
Kelly GA, Walkden-Brown SW, Kahn LP. No loss of production due to larval challenge in sheep given continuous anthelmintic treatment via a controlled release capsule. Vet Parasitol. 2012;183:274–83.
Khan MQ, Hayat S, Ilyas M, Hussain M, Iqbal Z. Effect of haemonchosis on body weight gain and blood values in sheep. Pak Vet J Pak. 1988.
Khan FA, Sanyal PK, Swarnkar CP, Singh D, Bhagwan PSK. Comparative anthelmintic activity of strategic sustained Low-level administration of Albendazole in feed pellets compared to single doses of closantel and tetramisole against natural ovine parasitic gastroenteritis. Trop Anim Health Prod. 1999;31:193–204.
Kimambo AE, MacRae JC, Walker A, Watt CF, Coop RL. Effect of prolonged subclinical infection with Trichostrongylus colubriformis on the performance and nitrogen metabolism of growing lambs. Vet Parasitol. 1988;28:191–203.
Knox MR, Steel JW. The effects of urea supplementation on production and parasitological responses of sheep infected with Haemonchus contortus and Trichostrongylus colubriformis. Vet Parasitol. 1999;83:123–35.
Kyriazakis I, Anderson DH, Oldham JD, Coop RL, Jackson F. Long-term subclinical infection with Trichostrongylus colubriformis: effects on food intake, diet selection and performance of growing lambs. Vet Parasitol. 1996;61:297–313.
Leathwick DM, Waghorn TS, Miller CM, Atkinson DS, Haack NA, Oliver A-M. Selective and on-demand drenching of lambs: Impact on parasite populations and performance of lambs. N Z Vet J. 2006;54:305–12.
Leyva V, Henderson AE, Sykes AR. Effect of daily infection with Ostertagia circumcincta larvae on food intake, milk production and wool growth in sheep. J Agric Sci. 1982;99:249–59.
Lindahl IL, Colglazier ML, Enzie FD, Turner JH, Whitmore GE, Wilson RL. Effect of management systems on the growth of lambs and development of internal parasitism. III. Field trials with lambs on soilage and pasture involving medication with N.F. And purified grades of phenothiazine. J Parasitol. 1970;56:991–9.
Liu SM, Smith TL, Briegel J, Murray A, Masters DG, Karlsson LJE, et al. Comparing productive performance of nematode resistant Merino sheep with non-selected control. Livest Prod Sci. 2005;97:117–29.
Louvandini H, Veloso CFM, Paludo GR, Dell’Porto A, Gennari SM, McManus CM. Influence of protein supplementation on the resistance and resilience on young hair sheep naturally infected with gastrointestinal nematodes during rainy and dry seasons. Vet Parasitol. 2006;137:103–11.
Louw J, Overberg Research Projects. III. A preventive worm control programme for sheep in the Ruens, in the winter rainfall region of South Africa. J S Afr Vet Assoc. 1989;60:186–90.
Louw J, Reinecke R, Overberg Research Projects VIII. The productivity of Merino ewes subjected to different internal parasite control programmes in the winter rainfall region of South Africa. J S Afr Vet Assoc. 1990;61:163–7.
Macchi C, Pomroy WE, Morris RS, Pfeiffer DU, West DM. Consequences of anthelmintic resistance on liveweight gain of lambs on commercial sheep farms. N Z Vet J. 2001;49:48–53.
Mage C, Reynal PH. Preventative effect of moxidectine oral drench against gastro-intestinal nematode in grazing lambs. Rev Med Veterinaire Fr. 1997;148.
Maingi N, Thamsborg SM, Gichohi VM, Munyua WK, Gathuma JM. The strategic use of closantel and albendazole in controlling naturally acquired gastrointestinal nematodes of sheep in the Kenya highlands. Vet Res Commun. 1997;21:547–57.
Maingi N, Munyua WK, Gichigi MN. Strategic use of moxidectin or closantel in combination with levamisole in the control of nematodes of sheep in the highlands of central Kenya. Acta Trop. 2002;84:93–100.
Marie-Magdeleine C, Boval M, Philibert L, Borde A, Archimède H. Effect of banana foliage (Musa x paradisiaca) on nutrition, parasite infection and growth of lambs. Livest Sci. 2010;131:234–9.
Marley CL, Fraser MD, Fychan R, Theobald VJ, Jones R. Effect of forage legumes and anthelmintic treatment on the performance, nutritional status and nematode parasites of grazing lambs. Vet Parasitol. 2005;131:267–82.
Marley CL, Fraser MD, Davies DA, Rees ME, Vale JE, Forbes AB. The effect of mixed or sequential grazing of cattle and sheep on the faecal egg counts and growth rates of weaned lambs when treated with anthelmintics. Vet Parasitol. 2006;142:134–41.
Martínez-Valladares M, Vara-Del Río MP, Cruz-Rojo MA, Rojo-Vázquez FA. Effect of a low protein diet on the resistance of Churra sheep to Teladorsagia circumcincta. Parasite Immunol. 2005;27:219–25.
Mavrogianni VS, Papadopoulos E, Fragkou IA, Gougoulis DA, Valasi I, Orfanou DC, et al. Administration of a long-acting antiparasitic to pre-pubertal ewe-lambs in Greece results in earlier reproductive activity and improved reproductive performance. Vet Parasitol. 2011;177:139–44.
Medina R, Sánchez A. Effect of supplementation with Leucaena leucocephala foliage on weight gain of drenched and non drenched sheep. Zootec Trop. 2006;24:55–68.
Miller JE, Burke JM, Terrill TH, Kearney MT. A comparison of two integrated approaches of controlling nematode parasites in small ruminants. Vet Parasitol. 2011;178:300–10.
Muenstermann S, Tome NR. Influence of regular tick and helminth control on productivity of small ruminants in the Lolgorien area, Narok district, Kenya. Trop Anim Health Prod. 1989;21:247–55.
Mugambi JM, Wanyangu SW, Bain RK, Owango MO, Duncan JL, Stear MJ. Response of dorper and red Maasai lambs to trickle Haemonchus contortus infections. Res Vet Sci. 1996;61:218–21.
Niezen JH, Robertson HA, Waghorn GC, Charleston WAG. Production, faecal egg counts and worm burdens of ewe lambs which grazed six contrasting forages. Vet Parasitol. 1998;80:15–27.
Ramírez-Restrepo CA, Barry TN, Pomroy WE, López-Villalobos N, McNabb WC, Kemp PD. Use of Lotus corniculatus containing condensed tannins to increase summer lamb growth under commercial dryland farming conditions with minimal anthelmintic drench input. Anim Feed Sci Technol. 2005;122:197–217.
Ríos-De Álvarez L, Greer AW, Jackson F, Athanasiadou S, Kyriazakis I, Huntley JF. The effect of dietary sainfoin (Onobrychis viciifolia) on local cellular responses to Trichostrongylus colubriformis in sheep. Parasitology. 2008;135:1117–24.
Rocha RA, Araújo JV, Amarante AFT. Efficacy of the nematode-trapping fungus Duddingtonia flagrans against infections by Haemonchus and Trichostrongylus species in lambs at pasture. J Helminthol. 2007;81:387–92.
Schichowski C, Moors E, Gauly M. Influence of weaning age and an experimental Haemonchus contortus infection on behaviour and growth rates of lambs. Appl Anim Behav Sci. 2010;125:103–8.
Sechi S, Giobbe M, Sanna G, Casu S, Carta A, Scala A. Effects of anthelmintic treatment on milk production in Sarda dairy ewes naturally infected by gastrointestinal nematodes. Small Rumin Res. 2010;88:145–50.
Strickland VJ. Dose response rate of garlic for the control of Haemonchus contortus in merino wethers and the subsequent sensory quality of the meat, Thesis. Curtin University: School of Agriculture and Environment; 2011.
Suarez VH, Cristel SL, Busetti MR. Epidemiology and effects of gastrointestinal nematode infection on milk productions of dairy ewes. Parasite. 2009;16:141–7.
Sykes AR, Coop RL, Angus KW. The influence of chronic Ostertagia circumcincta infection on the skeleton of growing sheep. J Comp Pathol. 1977;87:521–9.
Thomas RJ, George RW. Anthelmintic studies in fat lamb production. I. Autumn treatment of housed lambs with methyridine. Res Vet Sci. 1967;8:297–305.
Valderrábano J, Delfa R, Uriarte J. Effect of level of feed intake on the development of gastrointestinal parasitism in growing lambs. Vet Parasitol. 2002;104:327–38.
van Houtert MFJ, Barger IA, Steel JW, Windon RG, Emery DL. Effects of dietary protein intake on responses of young sheep to infection with Trichostrongylus colubriformis. Vet Parasitol. 1995;56:163–80.
Waller PJ, Axelsen A, Donald AD, Morley FHW, Dobson RJ, Donnelly JR. Effects of helminth infection on the pre-weaning production of ewes and lambs: comparison between safe and contaminated pasture. Aust Vet J. 1987;64:357–62.
Yacob HT, Mistre C, Adem AH, Basu AK. Parasitological and clinical responses of lambs experimentally infected with Haemonchus contortus (L3) with and without ivermectin treatment. Vet Parasitol. 2009;166:119–23.
Zacharias F, Guimarães JE, Araújo RR, Almeida MAO, Ayres MCC, Bavia ME, et al. Effect of homeopathic medicines on helminth parasitism and resistance of Haemonchus contortus infected sheep. Homeopathy. 2008;97:145–51.
Zaralis K, Tolkamp BJ, Houdijk JGM, Wylie ARG, Kyriazakis I. Consequences of protein supplementation for anorexia, expression of immunity and plasma leptin concentrations in parasitized ewes of two breeds. Br J Nutr. 2009;101:499–509.
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Kipper M, Andretta I, Monteiro SG, Lovatto PA, Lehnen CR. Meta-analysis of the effects of endoparasites on pig performance. Vet Parasitol. 2011;181:316–20.
Rinaldi L, Veneziano V, Morgoglione ME, Pennacchio S, Santaniello M, Schioppi M, et al. Is gastrointestinal strongyle faecal egg count influenced by hour of sample collection and worm burden in goats? Vet Parasitol. 2009;163:81–6.
Bishop SC, Stear MJ. The use of a gamma-type function to assess the relationship between the number of adult Teladorsagia circumcincta and total egg output. Parasitology. 2000;121:435–40.
Stear MJ, Abuagob O, Benothman M, Bishop SC, Innocent G, Kerr A, et al. Variation among faecal egg counts following natural nematode infection in Scottish Blackface lambs. Parasitology. 2006;132:275–80.
Good B, Hanrahan JP, Crowley BA, Mulcahy G. Texel sheep are more resistant to natural nematode challenge than Suffolk sheep based on faecal egg count and nematode burden. Vet Parasitol. 2006;136:317–27.
Jacobson C, Bell K, Forshaw D, Besier B. Association between nematode larvae and “low worm egg count diarrhoea” in sheep in Western Australia. Vet Parasitol. 2009;165:66–73.
Mair C, Matthews L, De Cisneros JPJ, Stefan T, Stear MJ. Multitrait indices to predict worm length and number in sheep with natural, mixed predominantly Teladorsagia circumcincta infection. Parasitology. 2015;142:773–82.
Kenyon F, Jackson F. Targeted flock/herd and individual ruminant treatment approaches. Vet Parasitol. 2012;186:10–7 [Special Issue: Novel Approaches to the Control of Helminth Parasites of Livestock].
Jackson F, Coop RL. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120:95–107.
Rose H, Rinaldi L, Bosco A, Mavrot F, de Waal T, Skuce P, et al. Widespread anthelmintic resistance in European farmed ruminants: a systematic review. Vet Rec. 2015;176:546.
Sayers G, Sweeney T. Gastrointestinal nematode infection in sheep – a review of the alternatives to anthelmintics in parasite control. Anim Health Res Rev. 2005;6:159–71.
Acknowledgements
Many thanks go to Mrs. B. Schneider for excellent help in retrieving studies and to Mrs. B. Otero Abad for translating studies.
Funding
This work was funded by the FP7 GLOWORM project—Grant agreement N° 109 288975CP-TP-KBBE.2011.1.3-04
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
PT and HH participated in the design of the study. FM performed the literature search and carried out the analysis under PT and HH guidance. FM drafted the manuscript and all authors contributed to and approved the final version.
Additional file
Additional file 1:
PRISMA 2009 Checklist. (Docx 30 KB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mavrot, F., Hertzberg, H. & Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: a systematic review and meta-analysis. Parasites Vectors 8, 557 (2015). https://doi.org/10.1186/s13071-015-1164-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-015-1164-z