Abstract
With the advancement in the egg industry sector, egg quality has assumed great significance in certain countries. Enhancements in the nutritional value of eggs may have direct affirmative consequences for daily nutrient intake and therefore for human health. Thus, affirmative improvement in egg quality boosts consumer preferences for eggs. Also, the improvement in eggshell quality can avoid the disposal of broken eggs and consequently economic losses. Therefore, poultry nutrition and mineral supplements have a significant impact on egg quality. Minerals are crucial in poultry feed for a number of biological processes, including catalytic, physiologic, and structural processes. For instance, they contribute to the biological processes necessary for forming and developing eggshells. To produce high-quality eggs for sale, diets must therefore contain the right amount of minerals. This review aims to highlight the role of both organic and inorganic minerals in improving egg quality, in addition to reviewing the interactions of mineral supplements with intestinal microbiota and subsequent effects on the egg quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Egg is a good source of nutrients such as vitamins A, D, E, and K, B2, B6, B12, as well as minerals including calcium, phosphorus, selenium, zinc, iron, and magnesium [97]. Egg quality is a key criterion for egg producers all over the world and has significant economic ramifications. While poor egg quality can result in losses, improving quality can help increase the value of the finished product. The nutrition and digestive health of hens are directly related to this criterion. The rearing system of the hens significantly affects the concentration of nutrients including elements in the egg. Eggs from organic farming systems have richer nutrients like magnesium, calcium, and zinc compared with eggs laid from caged hens [97].
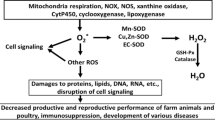
Mineral supplementation in diets of layer chickens contributes to boosting egg quality criteria. Minerals are important for laying hen diets because they contribute to the biochemical processes that support normal development and growth of body and eggs [82]. Based on the National Research Council [69], it can be concluded that the total Zn, Mn, Se, I, Fe, Mg, and Cu requirement for laying hens is around 29–45, 17–25, 0.06, 0.32–0.48, 38–60, 370–600, and 4–5 mg/kg. Also, the requirement of sodium and potassium is around 0.13–0.19% in the laying hen diets. Maintaining the optimal concentration of minerals in diets is necessary to obtain high egg quality for sale [59, 61]. Incorporating minerals in layers’ feed is essential to produce superior external traits of egg quality and to diminish the problem of limiting levels of minerals in hen commercial diets which based on the ingredients of soybean meal and corn with low concentrations of minerals [32]. Mineral contents in the whole egg and eggshell are quite variable, according to the dietary element form and dose, as well as other aspects such as physiological reactions, management practices, geographic area, and supplemented feed additives [11, 37]. The consideration of the mineral source, organic or inorganic, that requires for supplementation must be taken into account. Dietary organic mineral supplementation significantly improved internal egg quality besides the external one [86]. Minerals can also enhance certain physiological reactions including the immune reaction [70] and play crucial roles in the virulence of pathogens and the antimicrobial resistance of hosts [110]. For instance, guanosine 5′-monophosphate-chelated calcium and iron inhibited the growth of Salmonella gallinarum, boosted percentage of egg production, and decreased the proportion of both cracked eggs and broken eggs [70]. Therefore, from another side, supplemental minerals can be used to improve the egg quality criteria. This review was aimed to summarize the benefits of mineral supplementation for laying hen diets, factors interfering them and to fill in gaps in the knowledge of the influences of dietary minerals on laying hens’ egg quality (Fig. 1).
Minerals Sources in Diets of Laying Hens
Minerals are the main nutraceuticals required for physiological functions and optimum health. They are necessary as a part of the activator of enzymes and hormones for the eggshell formation and skeleton. The advanced knowing of the significance of minerals in reproduction and the changing of mineral levels in feed ingredients resulted in their supplementation in high quantities to the laying hens feed in the commercial sector with a considerable safety margin often surpassing the nutritional requirements [6, 7]. The inorganic sources are often incorporated into diets at higher proportions due to uncertainties associated with absorption (Araújo et al. 2008). However, this form results in a lack of nutritional balance and a potential for toxicological concerns [64], as well as a greater excretion of mineral than does the organic from [81, 102].
The bioavailability of minerals refers to the utilized portion by the organism, depending on their form (organic or inorganic). Inorganic minerals encompass oxides, sulfates, phosphates, and carbonates, while organic ones include proteinates, amino acids, polysaccharides, yeast, and complexes/chelates analogous to methionine. Feed-grade sources of trace minerals can differ greatly in purity. The variation in minerals’ bioavailability of these sources is also observed, wherein sulfates generally exhibit higher bioavailability than oxides [64]. Moreover, the element in some sources may need some enzymes to be available to the bird. For example, the bioavailability of P from plant sources is relatively limited, even though certain feed ingredients such as wheat and barley contain phytase which enhances the availability of P [14].
According to Suttle et al. (2010), bioavailability has been controlled by four steps: (1) the availability of such mineral to the absorptive enterocytes of mucosa that is affected by its form and its agonistic or antagonistic relations in the feed or in the gut; (2) the possibility of absorbed mineral transference through the mucosa layer (absorbability) which is depending upon the mucosa’s ability to uptake accessible minerals; (3) the ability of circulating minerals to avoid excretion through the kidneys or gut, and it measures the rate of mineral retention of the transferred ones (retainability); (4) the capacity of the minerals that assimilated into functional forms of retained ones which is influenced by the mineral absorbed forms and the site of its retention.
Organically chelated minerals showed superior bioavailability than those of inorganic metal salts [66, 101],therefore, using chelated or organic complexes of minerals in layers’ diets has been reported. The bioavailability of trace minerals determines their efficacy. Therefore, it appears that the additives of minerals in organic are more effective in supporting criteria of egg quality and egg production in laying hens [24, 32, 92, 106]. Furthermore, the complete substitution of inorganic mineral sources with organic ones in laying hens’ diets allowed the decrease in excreted minerals and did not impact egg production or the eggshell quality [20, 21]. Trace metal-amino acid complexes have the potential to mimic the mechanism by which trace elements are absorbed, making them available to animals more than inorganic forms [95]. However, such complexes must be robust enough to withstand natural dietary antagonists while still providing the complexed element to the tissues in a form that can be utilized [95]. The role of some minerals in enhancing egg quality will be shown in this review along with the difference between the effects of organic forms of minerals versus inorganic forms in laying diets (Table 1).
Effects of Minerals on Egg Quality
Zinc (Zn)
Zinc performs multiple functions in the formation of the skeletal system, the regulation of metabolic processes, the maintenance of antioxidant systems, and the enhancement of the immune response in poultry, as well as egg formation [6, 7]. Zinc is a constituent of several metalloenzymes, for example, carbonic anhydrase, which has crucial role in eggshell formation [116], where the crystal and texture morphologies of the eggshell are affected by carbonic anhydrase’s catalysis of carbon dioxide into bicarbonate ions [51]. Zinc is a vital trace mineral necessary for forming eggshells and can contribute to the process of calcium deposition as well as impact the structure and physical properties of the shell [51, 65]. Zn serves as a significant cofactor in carbonic anhydrase enzyme which is responsible for facilitating the hydration of circulating CO2 into HCO3 − and providing the precursor for eggshell carbonates [122]. Zinc is important during albumen deposition in the magnum, eggshell membrane formation in the isthmus, and shell formation in the uterus [12]. Augmented Zn supplementation may decline egg loss from breaks and cracks. Several research has demonstrated superior eggshell thickness and strength and less egg breakage in birds administrated with Zn in organic form or in a combination of inorganic and organic forms [40, 71, 92]. The addition of zinc methionine chelate to layer diets is recommended because it results in improved bone mechanical properties, without compromising the quality of eggshell at the end of the egg-laying cycle, indicating its positive impact in the overall maintenance of bone mineral reserves through the end of the egg-laying cycle [70].
Mixing ZnSO4 and Zn-amino acid (ZnAA) complex in broiler breeder feeds diminished the percentage of cracked eggs and enhanced the eggshell quality in comparable to the basal diet that contained ZnSO4 [40]. Swiatkiewicz and Korelski [96] illustrated that 50 and 100% substitution of Zn oxide with ZnAA complex increased the eggshell breaking strength in the aged hens (62:70 weeks of age) that received the basal diet enriched with 30 mg Zn/kg. Moreover, Manangi et al. [62] exhibited that using Zn–Cu–Mn chelated with hydroxy analog of methionine (20–5-20 and 40–10-40 ppm) as an organic Zn supplement augmented both eggshell strength and eggshell thickness in layers (44 to 80 weeks of age) comparing to those consumed the basal diet only or the basal diet enriched with sulfate salt of Zn–Cu–Mn (20–5-20, 40–10-40, and 80–10-80 ppm). Thus, Zn might ameliorate the adverse influence of age on the quality of eggshell.
Li et al. [50] stated that adding Zn-methionine (Zn-Met) in the basal diet at level of 100 ppm augmented egg’s Haugh unit (HU) and albumen height in comparable to the control that fed the basal diet supplemented with 80 ppm Zn as sulfate salt. Abd El-Hack et al. [1] illustrated that supplementing diets with 50, 75, or 100 ppm Zn-Met had considerable affirmative influence on the HU compared to the control with no Zn-Met supplements. These enhancements may be owing to the significance of Zn function in the egg formation. Besides, Amen and Al-Daraji [9] pointed out that the epithelium quality is affected by the deficiency of Zn owing to its role in the synthesis of protein.
As previously mentioned, zinc has an indirect impact on the secretion of the epithelium layer by altering its structure or directly influencing the secretion of the eggshell membranes. Also, it plays a vital role in the magnum and isthmus during egg albumin and eggshell membrane formation, respectively. Tabatabaie et al. [98] have demonstrated that HU and egg albumen percentage were elevated by dietary administration of 25 or 50 ppm organic Zn, in comparison to the control with no Zn administration. Nevertheless, Idowu et al. [41] declared no statistical changes in criteria of egg quality, except the values of HU, when layers consumed diets enriched with Zn-proteinate, Zn-carbonate, Zn-oxide, and Zn-sulfate at a level of 140 ppm. Also, zinc could maintain the quality of the stored eggs due to its ability to activate enzymatic antioxidant system in the egg. Organic zinc, at a concentration of 60 to 80 ppm, exerted a more pronounced influence in this regard compared to 80 ppm inorganic zinc [50]. According to Zhao et al. [144], zinc has an anti-oxidation function that makes birds more resistant to certain oxidative stresses. Furthermore, Zn can be used as a dietary supplement to control the negative effects brought on by various agents such as aflatoxicosis in addition to serving as a nutrient for birds [67]. Generally, eggshell traits of layers could be improved by the replacement of inorganic Zn with organic one, especially in older birds, but the mechanism was still unclear.
Selenium (Se)
Selenium revealed strong biological and nutritional influences in inducing physiology and production of poultry [6, 7] that were primarily mediated by the activity of selenoproteins [55, 93]. The dietary requirement of selenium for laying hens is comparatively low, at approximately 0.3 mg/kg [79]. Excess Se intake is toxic [101], and its inorganic form has limited biological availability, consequently its inclusion by high doses limits its utilization in poultry nutrition [120], as well as its emission to the environment is higher than the organic form [117]. Organisms take inorganic selenium and turn it into organic form [94]. Selenium-enriched yeast culture, bacterial Se, SeMet, OH-SeMet, Se-cysteine, and semethyl-Se cysteine are examples of selenium organic sources. Organic form of selenium can be transferred to eggs [18] with a higher rate than inorganic Se through its addition to diets of egg-laying hens. In addition to the enhanced efficacy in transferring to eggs, a better internal egg traits (e.g., HU) was documented as a result of the stimulation of selenoprotein, methionine sulfoxide reductase B enzyme, which is demanded for preventing protein oxidation and maintaining the albumen water-holding capacity [42, 102].
The dietary supplementation level of Se yeast (0.21, 0.36, and 0.43 ppm) increased Se levels in the whole egg, albumen, and yolk of laying ducks when compared to control feed that contained Se at level of 0.15 ppm [118]. A positive correlation (linear and quadratic) has been observed between the concentrations of Se in the egg and the level of Se-enriched yeast in the diet (0.3, 1.5, and 3 ppm) of 30-week-old laying hens [58]. However, after a 12-week feeding period, no considerable differences were noted in the fresh egg quality criteria (external and internal) between hens that received Se-enriched yeast diet and those fed a basal diet with no Se supplement.
Selenium is a vital component in many antioxidant enzymatic systems; consequently, the addition of Se might boost the activity of GSH-Px [107] and total antioxidant status of eggs when compared to the control with no Se addition [79].
Selenium can be used to prolong the storage period of the eggs due to its antioxidant effects. Saldanha et al. [85] stated that higher egg Se content allows maintaining the internal quality of egg during storage. Enriching layers diets with selenium markedly augmented its concentration in the egg, fatty acid composition, oxidative stability, and maintains quality of stored eggs such as yolk index [25, 35]. The greatest benefit regarding egg oxidative stability was seen by organic selenium addition in diets high in oxidized fat sources (Laika and Jahanian 2015).
Muhammad et al. (2021) recorded differences among organic (yeast and bacterial) and inorganic Se in gene expression of GPX1, GPX4, DIO1, DIO2, and SELW1, and they attributed it to the superior bioavailability of organic forms, which stimulates more selenoprotein gene expression [105]. It is notable that organic Se benefit is beyond just improved absorption. For instance, chelated Se to amino acids has much retention and incorporation into animal tissues [17] which might act as amino acid analogs for creating non-specific proteins. Additionally, the absorption and transportation of selenium in cleated form may be accomplished entirely to the target tissues, ultimately leading to higher bioavailability for metabolism when compared to inorganic selenium [17]. However, organic Se from different sources possesses different bioavailabilities in the body. So, the efficacy of organic Se source and level on layers’ performance and egg quality should be investigated.
Given that laying hens’ table eggs are used as food with high levels of selenium or as raw materials for food that are enriched with selenium [57], it follows that adding organic selenium in layer diets has remarkable practical importance for consumers.
In comparison to its inorganic counterpart, organic selenium is less toxic, has higher bioavailability and rates of retention and tissue accumulation, and possesses antioxidant properties. However, genome instability may result from excessive Se intake due to oxidative damage. Therefore, further examining the clinical and safety parameters of organic sources of Se and levels in healthy laying hens should be investigated.
Manganese (Mn)
Manganese plays a significant role in bone development of layers and is necessary for forming of eggshell and can positively affect the quality of eggshell [72, 104]. Manganese boosted eggshell strength by augmenting the process of biosynthesizing glycosaminoglycan (GAG) [121], which regulating mineral deposition eggshell and consequently determining the quality [22]. Supplementing the diet with Mn has the potential to enhance the quality of eggshells through the augmentation of GAG and uronic acids in the eggshell membrane [109]. The presence of Mn can influence the mechanical properties of the eggshell by modulating the formation of calcite crystals and the structure of the shell [59, 96]. Mn serves as a stimulator for enzymes involved in synthesizing mucopolysaccharides and glycoproteins, both of which play a crucial role in the creation of the organic matrix that forms the shell [86]. In the review of Olgun [72], the laying hens’ performance seems to be unaffected by dietary supplementation of inorganic Mn at dose of 200 ppm, but at lower doses, eggshell quality is improved. It appears that laying hens need about 90 ppm of Mn in their feed, and Mn-sulfate is more readily available than other forms of inorganic Mn, but lower than its organic forms. Mn is capable of activating enzymes that participate in the creation of glycoproteins and glycosaminoglycans, both of which participate in forming the shell organic matrix [92]. Sazzad et al. [87] found that eggshell thickness was augmented with the increase of dietary Mn addition up to 105 ppm (80 ppm MnO + 25 ppm basal diet). Fassani et al. [29] pointed out that elevating dose of dietary Mn supplementation (40 to 200 ppm) in the second production phase linearly augmented shell thickness and egg loss rate of laying hens. Mn deficiency causes a reduction in egg yield, enhanced the formation of eggs with a thin shell with translucent regions, and exhibited abnormal ultrastructure of the eggshell [33, 59].
Xiao et al. [108] noted that adding 100 ppm Mn in laying hen diets boosted eggshell characteristics (break strength, fracture toughness, and thickness), increasing uronic acid and glycosaminoglycan formation in the uterus, and consequently boosting the shell ultrastructure compared to basal diet with no Mn supplement. The use of Mn-Bioplex (organic form), at different levels in diet (15, 30, 45, 60, and 75 ppm) for 12 weeks in laying hens, increased the egg weight and decreased the of broken eggs %, when compared to the inorganic form [114]. Li et al. [49] studied the impact of dietary MnSO4 (60 ppm) as a control group and dietary Mn-methionine (Mn-Met) (20, 40, 60, and 80 ppm) on egg quality in laying hens and found that dietary Mn-Met treatments improved the egg internal traits (yolk color, albumen height, and HU), and the ultrastructure of the shell. Eggshell Mn levels were significantly increased by increasing Mn-Met addition, indicating that Mn content was distributed mainly in the eggshell. These studies demonstrate that the mechanical properties of eggshells were improved by dietary Mn.
Previous research has indicated that dietary supplementation of Mn with either organic or inorganic Mn can enhance the production and eggshell quality in aged laying hens and deficiency of Mn could potentially decline the shell matrix content of hexosamine and hexuronic acid [116]. This deficient in Mn causes a notable decrease in the levels of GAG and uronic acids within the shell membranes, but it does not in the calcified shell [109]. In addition, manganese deficiency in the diet led to diminish the expression of GlcAT-I mRNA and inhibit the synthesis of GAG in the uterus [109]. In the future, it might be necessary to reevaluate the requirements Mn for layers in order to achieve optimal eggshell characteristic.
Copper (Cu)
Copper plays a significant role in forming shell membranes, which influence eggshell texture, shape, and structure [34]. Copper has been detected at high levels in eggshell and its membranes. The deficiency of dietary copper has the potential to impact the shell membrane structure, texture, and shape, and the pigments of the eggshell [75]. Copper possesses the capability to impact the quality of the eggshell by stimulating the enzymes that involved in the processes of forming eggshell and its membrane, in addition to their ability to interact with calcite crystals during the eggshell formation process [33]. Copper is a vital element of the lysyl oxidase enzyme, which being important in forming collagen of eggshell membrane [48]. Copper ion is an active cofactor in the center of superoxide dismutase, an enzyme that inhibits free radical reactions. Furthermore, copper reduced yolk cholesterol content [54] and increased shell strength [76]. Dobrzañski et al. [23] confirmed that using organic Cu significantly increased the Cu content in eggs and eggshells, which indicates the higher availability of organic Cu compared to CuSO4. Lim and Paik [53] concluded that dietary supplementation of 100 ppm of methionine-Cu chelate can enhance eggshell quality compared to the control diet with 20 ppm of Cu. Olgun et al. [73] obtained eggs with lower percentage of broken eggs and heavier and thicker shells in birds supplemented with Cu (75 to 300 mg/kg feed) than no Cu supplementation. Pekel and Alp [76] stated that adding dietary organic copper exhibited no statistical changes in egg quality characteristics or yolk cholesterol. However, shell strength was decreased in eggs from layers supplemented with micronutrients including inorganic and organic Cu forms. Cu-lysine chelate supplementation in the drinking water (30 mg/L) of laying hens improved egg weight and albumen weight and height, with no changes in the shell strength parameters compared to control birds with no additives [20].
The deficiency of copper in hen diets leads to the occurrence of abnormalities in eggshell [75]. The eggshell of hens suffering from copper deficiency showcases an unconventional arrangement of the fibers of shell membrane, due to changes in the cross-links derived from lysine. This ultimately causes abnormalities in egg shape and physical properties [26]. Furthermore, the lack of copper as micronutrient, which being a component of numerous enzymes and their activators, can reduce egg yield and heighten the frequency of eggs with abnormalities in size and shape. For the best egg yield and quality, it is therefore recommended to determine the minimal effective dose, Cu form and source, and the timing of administration.
Iron (Fe)
Iron is an important cofactor of many enzymes and acts in the oxygen transporting and storing. It is involved in protein and energy metabolism, and improving antioxidant and immunity status [6, 7]. Iron participates in several important reactions such as oxygen transporting and storing, as well as energy supply and protein metabolism that controls egg production [60, 110]. Seo et al. [88] stated that providing diets with 100 ppm iron enhanced formation and breakdown of erythrocyte, and boosted egg color in brown-type hens through its role in the protoporphyrin production (the main shell brown pigment). Compared to the Fe depletion pretreatment group, Fe increases egg production and blood hemoglobin without changing the eggshell color and egg composition [105]. Xie et al. [111] revealed that dietary treatments with Fe-glycine (Gly) (20, 40, 60, and 80 ppm) improved internal egg quality (albumen height and HU) in comparable to control that received 60 ppm Fe as FeSO4. Nevertheless, dietary supplementation of Fe-Gly showed little effect on eggshell ultrastructure. Shell, yolk, and albumen contents of Fe heightened by dietary level Fe-Gly, where dietary Fe-Gly (60 or 80 ppm) showed greater content of iron in albumen and yolk than control. Bertechini et al. [19] noted higher concentrations of iron in the egg when the basal diet enriched with 80 ppm Fe as FeSO4. Also, Paik [74] detected that utilization of chelated Fe augmented the iron content of the yolk by up to 20%.
Egg enrichment with minerals could be achieved by dietary trace element supplementation. In addition, iron is one of the more significant trace elements for poultry, playing a vital role in egg production and quality. Therefore, in order to enhance these parameters, a suitable concentration of Fe must be provided.
Chromium (Cr)
Chromium has important functions in the metabolism and antioxidant status of birds [6, 7]. The legality of adding Cr to an animal’s diet differs depending on the country, the source of the Cr, and the kind of animal [90]. In 2016, the FDA approved the use of 200 ppb of chromium in the whole feed of broiler chickens [31]. The European Food Safety Authority recommended using 0.4–1.6 mg/kg of chromium methionine as a feed additive in full feeding mixes for all species (EFSA 2009).
Chromium has been detected as component in egg albumin and in protein cross-linking, which is required for creating albumen proteins and aides in transporting the ion to the egg albumin throughout the plumping process in the shell gland [44]. Moreover, it is hypothesized that the presence of chromium is essential for maintaining the physical properties of albumin [44]. Dietary chromium supplementation (400 ppb to the basal diet contained 1285 ppb) linearly increased the HU and shell thickness of layer chickens kept at low temperatures compared to the control diet [84]. Lien et al. [52] displayed no changes in the shell thickness in response to supplemental Cr picolinate at dose of 200, 400, or 800 ppb under thermally neutral conditions. Similarly, in 24- to 33-week-age brown-type layers, supplemental Cr picolinate in diets at 400 or 600 ppb had negligible effect on shell thickness and strength, yolk color, and HU compared to non-supplemented Cr group. Torki et al. [100] observed greater weight and eggshell thickness of heat-stressed hens that received 400 ppb of Cr picolinate compared to the Cr-non-supplemented control birds. Subsequently, Torki et al. [99] pointed out that adding Cr to diets of laying hens exposed to heat stress increased the HU and shell weight of eggs produced from laying hens after exposure to heat stress. Nevertheless, supplemental Cr exhibited insignificant impact on internal egg quality. These studies demonstrate that chromium supplementation is important to improve the shell quality of eggs produced at non-neutral temperatures.
Effect of Nano-minerals on Egg Quality
Lately, the utilization of nano-minerals has garnered considerable interest owing to their elevated bioavailability; hence, their integration in poultry diets is capable of augmenting performance and health [2, 27, 38, 118] (Table 2). Incorporating nano-minerals in diets positively affects egg quality of laying hens [47]. The incorporation of selenium nanoparticles into the layer feeds exhibited a remarkable impact on the egg laying productivity, egg quality, and the activity of oxidative enzymes [68]. Sirirat et al. [89] stated that using chromium picolinate nanoparticles has the potential to enhance the quality of eggs. The latest authors revealed that layer-fed diets containing 500 and 3000 ppb nanometric chromium picolinate produced eggs with low yolk weight percentage. Laying hens fed on diets supplemented with zinc oxide nanoparticles achieved the highest significant improvement in egg shell quality, egg index, yolk and white quality, and HU compared to the control group [4]. The enhancement observed can potentially be attributed to the exceptional characteristics of nano-minerals, e.g., their remarkable biological availability, higher surface activity, improved mobility, increased solubility, and enhanced cellular uptake [63, 115].
Interaction of Dietary Constituents with Minerals and Subsequent Effects on Egg Quality
The inorganic trace mineral absorption is limited by the interactions with other feed nutrients including the other trace minerals [111]. The mineral form and quantity determine how much mineral is deposited in the egg contents [13]. For example, chickens exposed for a long time to Cr showed drops in serum levels of Ca, Fe, Mg, Zn, and Cu [122]. Zinc is considered the first limiting trace mineral among others, i.e., Cu, Fe, Mn, and Zn, so supplementing Zn-deficient diets with organic form of these minerals alone or in combination led to nonsignificant change in bird performance and were primarily excreted [15]. Zn-methionine exhibits superior bioavailability compared to inorganic one owing to its stability and withstands interference against disruption caused by other ligands in the gastrointestinal system [51]. The availability of Cu could be decreased by consuming more Zn and Fe, and a lack of Cu would cause abnormalities in shell formation [33].
The most significant source of organic P, inositol phosphate 6 (InsP6), is regarded as an anti-nutritional factor because it forms complexes with minerals and inhibits the absorption of cations in poultry [108]. The high-calcium-content diets of layers, which is necessary for forming eggshells, may inhibit phytate-degrading enzymes, reducing the hen’s access to phosphorus [39] and additionally resulting in the inappropriateness of phytase superdosing in mixed diets of layers (Skřivan et al. 2018).
On the other hand, the synergy between minerals and other dietary supplements, e.g., phenolic compounds and essential oils that present in plant extracts, can maximize the effects of minerals. The chelation among these compounds augments the absorbed minerals and their utilization; this shows that the two together can be more effective than either used alone [91].
Dietary incorporation of inorganic mineral with 100 ppm of rosemary oil enhanced yolk color and the relative weight of egg albumen of laying hens [32]. Additionally, the advantages of using essential oils on digestibility [8] led to more absorption of nutrients, including minerals, from the feed, and enhancing the quality of eggs. A further strategy to lessen the negative effects of P, Cu, and Zn on the environment is to increase mineral digestibility using exogenous enzymes. For instance, exogenous phytase added to the feed improves the piglets’ ability to digest zinc [28]. Additionally, this would require less mineral supplementation ([28]; EFSA 2016). Therefore, research into the adverse effects and interferences of trace minerals, particularly in mixtures, on digestion, absorption, and utilization, should be done in order to spot nutritional solutions to maximize egg yield and quality while reducing the environmental pollution.
The Interaction of Intestinal Microbiota with Minerals and Subsequent Effects on Egg Quality
A complex community of numerous microorganisms makes up the gastrointestinal tract (GIT) microbiota of laying hens. This microbial community creates the intestinal microbial barrier, which is crucial for nutrient digestion and absorption, gut health, and the immune system [5, 45]. There are differences in microbial composition that can be seen related to feed and its nutrients (Leeming et al. 2021). A two-way relationship exists between trace minerals and the digestive tract microorganisms. Through their ability to directly affect the absorption of minerals within the digestive tract and the production of important enzymes like phytase, which assist in releasing minerals from the feed, microorganisms of gastrointestinal tract exert a profound influence on the metabolic processes of trace minerals [36]. Additionally, an acidic medium and strong gastrointestinal functions are necessary dissolving calcium and phosphorus to make them more absorbable and utilizable [112]. On the other way, the gut microbial content and role might be changed by trace minerals, and they could also compartmentalize metabolic inflammation [16].
Mineral Supplementation May Change Gut Microbiota Composition, Resulting in Improved Egg Quality
According to Dong et al. [24], the harmful cecal microbiota (Barnesiellaceae and Clostridia) significantly decreased in layers that consumed inorganic (full dose) or organic (half dose) Cu, Mn, Zn, and Fe when compared to control. Furthermore, they demonstrated an association between the gastrointestinal microbiota and the genes’ expression associated with the uterus (such as vocalyxin-32 (OCX-32), osteopontin (OPN), and ALAS). As a result, distinct microbiota was negatively correlated with various eggshell characteristics, including absolute and relative weight, quality, and breaking strength. Hence, the integrity gastrointestinal tract and higher absorbability of trace minerals may be related to the decrease in the load of dangerous Barnesiellaceae and Clostridiales bacteria. But the thickness and color of the eggshell, however, were not connected to any cecal bacteria [24]. According to Abdelqader et al. [3], supplementing diets of laying hens with Bacillus subtilis exhibited higher relative weight of the eggshell and higher calcium retention, along with a decline in the load of intestinal Clostridium. Adding organic yeast Se to laying hen feed (0.3 mg/kg) increased the diversity of intestine microbes in pre- or post-challenge with S. Enteritidis, maintained gastrointestinal well-being by raising the load of anti-inflammatory microbes (Barnesiella and Bacteroidales), and enhanced the egg quality [43]. According to the authors, selenium supplementation exhibited considerable influence on how the content of the gut’s microbes responded to antioxidants or immune stimulation. In order to enhance the quality of eggshells and hen health, intestinal bacterial regulation can therefore be crucial. However, Roth et al. [83] demonstrated that the distribution of the microbiota and predicted functions in two breeds of high production laying hen were unaffected by a 20% reduction in dietary Ca and P.
Conclusion
Using organic minerals in the nutrition of laying hens has a wide range of advantages that apply to different product categories. To achieve the beneficial potential of supplementing feeds of laying hens with mineral, it is required to pay attention to the different supplementation doses, the different sources, their bioavailability, and transport as well as understanding the mechanisms to obtain much greater bioavailability. The possibility of using reduced mineral doses from organic sources could improve egg quality and reduce Cu, Zn, and P emissions into the environment. However, the output of mineral supplementation in laying hen diets is affected by the strain, breed, age, or duration of supplementation, and interactions between trace elements and feed additives. Only the optimal mineral supplementation would support high productive and reproductive performance and egg quality (internal and external traits). To understand the mechanisms of augmentation or antagonistic effects and achieve the greatest beneficial effects for laying hens, it is possible to focus on the interaction of probiotics or the microbiome in general with mineral supplementation. In addition, the efficacy within the different types of organic minerals (complexes/chelates) and their mechanisms in laying hens should be highlighted in further studies. Some trace elements have antioxidants or immune-stimulating activities, such as zinc, selenium, copper, and iron have direct and indirect positive effects on laying birds. They are not only used as nutrients to support egg performance but they also play a significant protective role against the negative effects of stressful situations, such as illnesses that affect egg production and egg quality, or they can strengthen various body systems against these agents. Data concerning the organic minerals’ impact on egg quality traits are quite variable and molecular mechanisms of organic mineral actions need further research. In addition, substantially increased cost, in comparison to inorganic minerals, is an important factor to be considered in commercial poultry production. Therefore, it is crucial to assess the mineral requirements for laying hens to be advantageous on a variety of aspects of the bird’s body under healthy or disease conditions by choosing the ideal dose and form without having negative effects on the bird, its products, and subsequently the consumer, or the environment.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abd El-Hack ME, Alagawany M, Amer SA, Arif M, Wahdan KM, El-Kholy MS (2018) Effect of dietary supplementation of organic zinc on laying performance, egg quality, and some biochemical parameters of laying hens. J Anim Physiol Anim Nutr 102(2):e542–e549
Abdelnour SA, Alagawany M, Hashem NM, Farag MR, Alghamdi ES, Hassan FU, Bila RM, Elnesr SS, Dawood MAO, Nagadi SA, Elwan HAM, Almasoudi AG, Attia YA (1916) Nanominerals fabrication methods, benefits and hazards, and their applications in ruminants with special reference to selenium and zinc nanoparticles. Animals 2021:11. https://doi.org/10.3390/ani11071916
Abdelqader A, Irshaid R, Al-Fataftah AR (2013) Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop Anim Health Prod 45:1017–1024
Abou-Ashour A, Zanaty G, El-Naga Manal Abou, Darwish A, Hussein Eman (2023) Utilization of dietary zinc oxide nanoparticles on productive and physiological performance of local laying hens. Egyptian J Nutr Feeds 26(3):429–442
Agus A, Planchais J, Sokol H (2018) Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23:716–724. https://doi.org/10.1016/j.chom.2018.05.003
Alagawany M, Elnesr SS, Farag MR, Tiwari R, Yatoo MI, Karthik K, Dhama K (2021a) Nutritional significance of amino acids, vitamins, and minerals as nutraceuticals in poultry production and health–a comprehensive review. Vet Quarterly 41(1):1–29
Alagawany M, Qattan SYA, Attia YA, El-Saadony MT, Elnesr SS, Mahmoud MA, Madkour M, Abd El-Hack ME, Reda FM (2021b) Use of chemical nano-selenium as an antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbes. Animals 11:3027
AL-Kassie G, Abd-AL-Jaleel R, Mohseen AM (2011) The effect of a mixture of anise and rosemary on broiler performance. Agric Biol J N Am 2:1279–1282
Amen MH, Al-Daraji HJ (2011) Effect of dietary supplementation with different levels of zinc on sperm-egg penetration and fertility traits of broiler breeder chicken. Pak J Nutr 10(11):1083–1088
Andejekovic M, Camp JV, Meulenaer B, Depaemelaere G, Socaciu C, Verloo M, Verhe R (2006) Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem 98:23–31
Bäckermann S, Ternes W (2008) Changes in mineral contents of hens’ egg yolk fractions due to biological and technological influences. Archiv für Geflügelkunde 72(5):213–220
Bahakaim A, Abdel Magied H, Osman S, Omar A, Abdelmalak NY, Ramadan N (2014) Effect of using different levels and sources of zinc in layer’s diets on egg zinc enrichment. Egypt Poult Sci J 34(1):39–56
Bai S, Jin G, Li D, Ding X, Wang J, Zhang K, Zeng Q, Ji F, Zhao. (2017) Dietary organic trace minerals level influences eggshell quality and minerals retention in hens. Ann Anim Sci 2017(17):503–515. https://doi.org/10.1515/aoas-2016-0074
Baker DH, HH Stein (2013) Bioavailability of minerals and vitamins in feedstuffs. In: Sustainable Swine Nutrition Chiba LI (Ed). Wiley-Blackwell: Oxford, UK. pp 341–364
Bao YM, Choct M, Iji PA, Bruerton K (2010) Trace mineral interactions in broiler chicken diets. Br Poult Sci 51(1):109–117. https://doi.org/10.1080/00071660903571904
Barra NG, Anhê FF, Cavallari JF, Singh AM, Chan DY, Schertzer JD (2021) Micronutrients impact the gut microbiota and blood glucose. J Endocrinol 250:R1-21. https://doi.org/10.1530/JOE-21-0081
Bartolini D, Sancineto L, Fabro de Bem A, Tew KD, Santi C, Radi R, Toquato P, Galli F (2017) Chapter Ten – Selenocompounds. In: Kenneth D Tew, Francesco Galli (eds) Cancer Therapy: An Overview, Advances in Cancer Research Vol 136. Academic Press pp. 259-302
Bennett DC, Cheng KM (2010) Selenium enrichment of table eggs. Poult Sci 89(10):2166–2172
Bertechini AG, Fassani EJ, Fialho ET, Spadoni JA (2000) Iron supplementation for commercial laying hens in the second cycle of production. Revista Brasileira de Ciencia Avícola 2(3):267–272
Brodacki A, Batkowska J, Stępniowska A, Blicharska E, Drabik K (2018) Quality and mineral composition of eggs from hens supplemented with copper-lysine chelate. Arch Anim Breed 61(1):109–113
Carvalho LSS, Rosa DRV, Litz FH, Fagundes NS, Fernandes EA (2015) Effect of the inclusion of organic copper, manganese, and zinc in the diet of layers on mineral excretion, egg production, and eggshell quality. Braz J Poultry Sci 17:87–92
Chien YC, Hincke MT, Vali H, McKee MD (2008) Ultrastructural matrix–mineral relationships in avian eggshell, and effects of osteopontin on calcite growth in vitro. J Struct Biol 163(1):84–99
Dobrzañski Z, Korczyñski M, Chojnacka K, Górecki H, Opaliñski S (2008) Influence of organic forms of copper, manganese, and iron on bioaccumulation of these metals and zinc in laying hens. J Elem 13(3):309–319
Dong Y, Zhang K, Han M, Miao Z, Liu C, Li J (2022) Low level of dietary organic trace minerals improved egg quality and modulated the status of eggshell gland and intestinal microflora of laying hens during the late production stage. Front Vet Sci 9:920418. https://doi.org/10.3389/fvets.2022.920418
Dos Reis JH, Gebert RR, Fortuoso BF, Dos Santos DS, Souza CF, Baldissera MD, Tavernari FC, Boiago MM, Paiano D, Da Silva AS (2019) Selenomethionine as a dietary supplement for laying hens: impacts on lipid peroxidation and antioxidant capacity in fresh and stored eggs. J Food Biochem 43(8):e12957. https://doi.org/10.1111/jfbc.12957
El-Husseiny O, Fayed SA, Omara II (2009) Response of layer performance to iron and copper pathway and their interactions. Aust J Basic Appl Sci 3(4):4199–4213
El-Seedi HR, El-Shabasy RM, Khalifa SAM, Saeed A, Shah A, Shah R, Iftikhar FJ, Abdel-Daim MM, Omri A, Hajrahand NH, Sabir JSM, Zou XB, Halabi MF, Sarhan W, Guo WS (2019) Metal nanoparticles fabricated by green chemistry using natural extracts: biosynthesis, mechanisms, and applications. RSC Adv 9(42):24539–59
European Food Safety Authority (EFSA) Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (2014) Scientific Opinion on the potential reduction of the currently authorised maximum zinc content in complete feed. EFSA J 12(5):3668–3745
Fassani ÉJ, Bertechini AG, De Oliveira BL, Gonçalves TDM, Fialho ET (2000) Manganese in nutrition of leghorn hens in the second cycle of production. Ciência e Agrotecnologia 24(2):468–478
Fernandes JIM, Murakami AE, Sakamoto MI, Souza LMG, Malaguido A, Martins EN (2008) Effects of organic mineral dietary supplementation on production performance and egg quality of white layers. Braz J Poultry Sci 10:59–65
Food and Drug Administration (FDA) (2020) Food additives permitted in feed and drinking water of animals; chromium propionate. Fed Reg 85:48650–48651
Garcia ERDM, Chaves NR, Oliveira CAL, Kiefer C, Melo EP (2019) Performance and egg quality of laying hens fed with mineral sources and rosemary oil. An Acad Bras Ciênc 91(2):e20180516
Gheisari AA, Sanei A, Samie A, Gheisari MM, Toghyani M (2011) Effect of diets supplemented with different levels of manganese, zinc, and copper from their organic or inorganic sources on egg production and quality characteristics in laying hens. Biol Trace Elem Res 142:557–571. https://doi.org/10.1007/s12011-010-8779-x
Gou Z, Fan Q, Li L, Wang Y, Lin X, Cui X, Jiang S (2021) High dietary copper induces oxidative stress and leads to decreased egg quality and reproductive performance of Chinese Yellow broiler breeder hens. Poult Sci 100(3):100779
Gravena RA, Marques RH, Roccon J, Picarelli J, Hada FH, Silva JDTD, Moraes VMBD (2011) Egg quality during storage and deposition of minerals in eggs from quails fed diets supplemented with organic selenium, zinc, and manganese. Revista Brasileira de Zootecnia 40:2767–2775
Hadadi N, Berweiler V, Wang H, Trajkovski M (2021) Intestinal microbiota as a route for micronutrient bioavailability. Curr Opin Endocr. Metab Res 20:100285. https://doi.org/10.1016/j.coemr.2021.100285
Hashish SM, Abdel-Samee LD, Abdel-Wahhab MA (2012) Mineral and heavy metals content in eggs of local hens at different geographic areas in Egypt. Glob Vet 8(3):298–304
Hassan HA, Arafat AR, Farroh KY, Bahnas MS, El-Wardany I, Elnesr SS (2022) Effect of in ovo copper injection on body weight, immune response, blood biochemistry and carcass traits of broiler chicks at 35 days of age. Anim Biotechnol 33(6):1134–1141
Hofmann T, Schmucker S, Sommerfeld V, Huber K, Rodehutscord M, Stefanski V (2021) Immunomodulatory effects of dietary phosphorus and calcium in two strains of laying hens. Animals 11:129. https://doi.org/10.3390/ani11010129
Hudson BP, Dozier Iii WA, Wilson JL, Sander JE, Ward TL (2004) Reproductive performance and immune status of caged broiler breeder hens provided diets supplemented with either inorganic or organic sources of zinc from hatching to 65 wk of age. J Appl Poultry Res 13(2):349–359
Idowu OMO, Ajuwon RO, Oso AO, Akinloye OA (2011) Effects of zinc supplementation on laying performance, serum chemistry, and Zn residue in tibia bone, liver, excreta, and eggshell of laying hens. Int J Poult Sci 10(3):225–230
Jlali M, Briens M, Rouffineau F, Mercerand F, Geraert PA, Mercier Y (2013) Effect of 2-hydroxy-4-methylselenobutanoic acid as a dietary selenium supplement to improve the selenium concentration of table eggs. J Anim Sci 91(4):1745–1752
Kang R, Wang W, Liu Y, Huang S, Xu J, Zhao L, Zhang J, Ji C, Wang Z, Hu Y, Ma Q (2022) Dietary selenium sources alleviate immune challenge induced by Salmonella enteritidis potentially through improving the host immune response and gut microbiota in laying hens. Front Immunol 13:928865. https://doi.org/10.3389/fimmu.2022.928865
Khan RU, Shabana N, Kuldeep D, Mani S, Ruchi T, Jeon G, Laudadio V, Tufarelli V (2014) Modes of action and beneficial applications of chromium in poultry nutrition, production and health: a review. Int J Pharmacol 10(7):357–367
Khan S, Moore RJ, Stanley D, Chousalkar KK (2020) The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl Environ Microbiol 86(3):e00600-e620. https://doi.org/10.1128/AEM.00600-20
Kim CH, Jeong SH, Lim SJ, Cheon SN, Kim K, Chun J, Jeon J (2022) Effect of organic or inorganic mineral premix in the diet on laying performance of agedlaying hens and eggshell quality. Animals 12:2378 https://doi.org/10.3390/ani12182378
Konkol D, Wojnarowski K (2018) The use of nanominerals in animal nutrition as a way to improve the composition and quality of animal products. J Chem 2018:1–7. https://doi.org/10.1155/2018/5927058
Leeson S, Summers JD (2001) Nutrition of the chicken, 4th edn. University books, Guelph, p 591
Li LL, Zhang NN, Gong YJ, Zhou MY, Zhan HQ, Zou XT (2018) Effects of dietary Mn-methionine supplementation on the egg quality of laying hens. Poult Sci 97(1):247–254
Li LL, Gong YJ, Zhan HQ, Zheng YX, Zou XT (2019) Effects of dietary Zn-methionine supplementation on the laying performance, egg quality, antioxidant capacity, and serum parameters of laying hens. Poult Sci 198(2):923–931. https://doi.org/10.3382/ps/pey440
Li L, Miao L, Zhu M, Wang L, Zou X (2019) Dietary addition of zinc-methionine influenced eggshell quality by affecting calcium deposition in eggshell formation of laying hens. Br J Nutr 122:961–973. https://doi.org/10.1017/S000711451900206X
Lien TF, Chen SY, Shiau S, Froman D, Hu CY (1996) Chromium picolinate reduces laying hen serum and egg yolk cholesterol. Prof Animal Scient 12:77–80
Lim HS, Paik IK (2006) Effects of dietary supplementation of copper chelates in the form of methionine, chitosan, and yeast in laying hens. Asian-Australas J Anim Sci 19:1174–1178
Lim KS, You SJ, An BK, Kang CW (2006) Effects of dietary garlic powder and copper on cholesterol content and quality characteristics of chicken eggs. Asian-Australas J Anim Sci 19(4):582–586
Liu CP, Fu J, Lin SL, Wang XS, Li S (2014) Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 159(1–3):192–198
Londero A, Pires Rosa A, Golin Luiggi F, Oliveira Fernandes M, Guterres A, Moura S, Hettwer Pedroso N, Santos N (2020) Effect of supplementation with organic and inorganic minerals on the performance, egg and sperm quality and hatching characteristics of laying breeder hens. Anim Reprod Sci 215:106309. https://doi.org/10.1016/j.anireprosci.2020.106309
Lu J, Qu L, Ma M, Li YF, Wang XG, Yang Z, Wang KH (2020) Efficacy evaluation of selenium-enriched yeast in laying hens effects on performance, egg quality, organ development, and selenium deposition. Poult Sci 99(11):6267–6277 https://doi.org/10.1016/j.psj.2020.07.041
Lu J, Qu L, Shen MM, Wang XG, Guo J, Hu YP, Dou TC, Wang KH (2019) Effects of high-dose selenium-enriched yeast on laying performance, egg quality, clinical blood parameters, organ development, and selenium deposition in laying hens. Poult Sci 198(6):2522–2530. https://doi.org/10.3382/ps/pey597
Mabe I, Rapp C, Bain MM, Nys Y (2003) Supplementation of a corn-soybean meal diet with manganese, copper, and zinc from organic or inorganic sources improves eggshell quality in aged laying hens. Poult Sci 82(12):1903–1913
Mackenzie EL, Iwasaki K, Tsuji Y (2008) Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal 10(6):997–1030
Mahmoud BY, Semida DA, Elnesr SS, Elwan H, El-Full EA (2023) Approaches of egg decontamination for sustainable food safety. Sustain 15(1):464
Manangi MK, Vazques-Anon M, Richards JD, Carter S, Knight CD (2015) The impact of feeding supplemental chelated trace minerals on shell quality, tibia breaking strength, and immune response in laying hens. J Appl Poultry Res 24(3):316–326
Michalak I, Dziergowska K, Alagawany M, Farag MR, El-Shall NA, Tuli HS, Emran TB, Dhama K (2022) The effect of metal-containing nanoparticles on the health, performance and production of livestock animals and poultry. Vet Quart 42(1):68–94. https://doi.org/10.1080/01652176.2022.2073399
Mikulewicz M, Chojnacka K, Kawala B, Gredes T (2017) Trace elements in living systems: from beneficial to toxic effects. Biomed Res Int 8297814:2 https://doi.org/10.1155/2017/8297814
Min YN, Liu FX, Qi X, Ji S, Ma SX, Liu X, Gao AY (2018) Effects of methionine hydroxyl analog chelated zinc on laying performance, eggshell quality, eggshell mineral deposition, and activities of Zn-containing enzymes in aged laying hens. Poult Sci 97(10):3587–3593
Mohammadi V, Ghazanfari S, Mohammadi-Sangcheshmeh A, Nazarian MH (2015) Comparative effects of zinc-nano complexes, zinc-sulfate, and zinc-methionine on performance in broiler chickens. Br Poult Sci 56(4):486–493
Mughal MJ, Peng X, Kamboh AA, Zhou Y, Fang J (2017) Aflatoxin B1 induced systemic toxicity in poultry and rescue effects of selenium and zinc. Biol Trace Elem Res 178(2):292–300. https://doi.org/10.1007/s12011-016-0923-9
Nabi F, Arain MA, Hassan F, Umar M, Rajput N, Alagawany M, Syed SF, Soomro J, Somroo F, Liu J (2020) Nutraceutical role of selenium nanoparticles in poultry nutrition: a review. World’s Poult Sci J 76(3):1–13
NRC National Research Council (1994) Nutrient Requirement for Poultry. Ninth Revised Ed. National Academy Press, USA
Noh HJ, Kim H, Heo SJ, Cho HH, Koh HB (2017) Guanosine 5′-monophosphate-chelated calcium and iron feed additives maintains egg production and prevents Salmonella Gallinarum in experimentally infected layers. J Vet Sci 18(3):291–297. https://doi.org/10.4142/jvs.2017.18.3.291
Ogbuewu IP, Mbajiorgu CA (2022) Meta-analysis of zinc supplementation on laying performance, egg quality characteristics, and blood zinc concentrations in laying hens. Biol Trace Elem Res 200(12):5188–5204. https://doi.org/10.1007/s12011-021-03080-8
Olgun O (2017) Manganese in poultry nutrition and its effect on performance and eggshell quality. World’s Poult Sci J 73(1):45–56
Olgun O, Yazgan O, Cufadar Y (2012) Effects of boron and copper dietary supplementation in laying hens on egg shell quality, plasma and tibia mineral concentrations and bone biomechanical properties. Revue de Médecine Vétérinaire 163(7):335–342
Paik IK (2001) Application of chelated minerals in animal production. Asian Australas J Anim Sci 14:191–198
Palanisamy V, Pc S, Pineda L, Han Y (2023) Effect of supplementing hydroxy trace minerals (Cu, Zn, and Mn) on egg quality and performance of laying hens under tropical conditions. Anim Biosci 36(11):1709–1717
Pekel AY, Alp M (2011) Effects of different dietary copper sources on laying hen performance and egg yolk cholesterol. J Appl Poultry Res 20(4):506–513
Qiu J, Lu X, Ma L, Hou C, He J, Liu B, Yu DG, Lin G, and J. Xu, (2020) Low-dose of organic trace minerals reduced fecal mineral excretion without compromising performance of laying hens. Asian Australas J Anim Sci 33(4):588–596. https://doi.org/10.5713/ajas.19.0270
Qiu JL, Zhou Q, Zhu JM, Lu XT, Liu B, Yu DY, Lin G, Ao T, Xu JM (2020) Organic trace minerals improve eggshell quality by improving the eggshell ultrastructure of laying hens during the late laying period. Poult Sci 99:1483–1490
Qiu K, Zheng JJ, Obianwuna UE, Wang J, Zhang HJ, Qi GH, Wu SG (2021) Effects of dietary selenium sources on physiological status of laying hens and production of selenium-enriched eggs. Front Nutr 8 https://doi.org/10.3389/fnut.2021.726770.
Ramos-Vidales D, Gómez-Verduzco G, Cortes-Cuevas A, Del Río-García JC, Fernández-Tinoco S, Chárraga-Aguilar S, Ávila-González E (2019) Organic trace minerals on productive performance, egg quality and immune response in Bovans white laying hens. J Anim Physiol Anim Nutr 103(5):1484–1491. https://doi.org/10.1111/jpn.13156
Reda FM, El-Saadony MT, El-Rayes TK, Attia AI, El-Sayed SAA, Ahmed SYA, Madkour M, Alagawany M (2021) Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital J Anim Sci 20(1):324–335
Richards JD, Zhao J, Harrell RJ, Atwell CA, Dibner JJ (2010) Trace mineral nutrition in poultry and swine. Asian Australas J Anim Sci 23(11):1527–1534
Roth C, Sims T, Rodehutscord M, Seifert J, Camarinha-Silva A (2022) The active core microbiota of two high-yielding laying hen breeds fed with different levels of calcium and phosphorus. Front Physiol 13:951350. https://doi.org/10.3389/fphys.2022.951350
Sahin N, Onderci M, Sahin K (2002) Effects of dietary chromium and zinc on egg production, egg quality, and some blood metabolites of laying hens reared under low ambient temperature. Biol Trace Elem Res 85(1):47–58
Saldanha ESPB, Garcia EA, Pizzolante CC, Faittarone ABG, da Sechinato A, Molino AB, Laganá C (2009) Effect of organic mineral supplementation on the egg quality of semi-heavy layers in their second cycle of lay. Braz J Poultry Sci 11(4):215–222
Saleh AA, Eltantawy MS, Gawish EM, Younis HH, Amber KA, Abd El-Moneim AEME, Ebeid TA (2020) Impact of dietary organic mineral supplementation on reproductive performance, egg quality characteristics, lipid oxidation, ovarian follicular development, and immune response in laying hens under high ambient temperature. Biol Trace Elem Res 195(2):506–514
Sazzad HM, Bertechini AG, Nobre PTC (1994) Egg production, tissue deposition and mineral metabolism in two strains of commercial layers with various levels of manganese in diets. Anim Feed Sci Technol 46(3–4):271–275
Seo YM, Shin KS, Rhee AR, Chi YS, Han J, Paik IK (2010) Effects of dietary Fe-soy proteinate and MgO on egg production and quality of eggshell in laying hens. Asian Australas J Anim Sci 23(8):1043–1048
Sirirat N, Lu JJ, Hung ATY, Lien TF (2013) Effect of different levels of nanoparticles chromium picolinate supplementation on performance, egg quality, mineral retention, and tissues minerals accumulation in layer chickens. J Agric Sci 5(2):150–159
Spears JW (2019) Boron, chromium, manganese, and nickel in agricultural animal production. Biol Trace Elem Res 188(1):35–44. https://doi.org/10.1007/s12011-018-1529-1
Stef DS, Gergen I (2012) effect of mineral-enriched diet and medicinal herbs on Fe, Mn, Zn, and Cu uptake in chicken. Chem Cent J 6:2–9
Stefanello C, Santos TC, Murakami AE, Martins EN, Carneiro TC (2014) Productive performance, eggshell quality, and eggshell ultrastructure of laying hens fed diets supplemented with organic trace minerals. Poult Sci 93(1):104–113
Sun B, Wang R, Li J, Jiang Z, Xu S (2011) Dietary selenium affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res 143(3):1516–1523. https://doi.org/10.1007/s12011-011-8995-z
Sun X, Yue S, Qiao Y, Sun Z, Li H (2020) Dietary supplementation with selenium enriched earthworm powder improves antioxidative ability and immunity of laying hens. Poultry Sci 99(11):5344–5349. https://doi.org/10.1016/j.psj.2020.07.030
Suttle NF (2010) Mineral nutrition of livestock. Cabi
Swiatkiewicz S, Koreleski J (2008) The effect of zinc and manganese source in the diet for laying hens on eggshell and bones quality. Vet Med 53(10):555–563
Szymanek E, Andraszek K, Banaszewska D, Drabik K, Batkowska J (2019) Content of selected inorganic compounds in the eggs of hens kept in two different systems: organic and battery cage. Arch Anim Breed 62:431–436. https://doi.org/10.5194/aab-62-431-2019
Tabatabaie MM, Aliarabi H, Saki A, Ahmadi A, Siyar SH (2007) Effect of different levels and sources of zinc on egg quality and layer performance. Pak J Biol Sci 10(19):3476–3478
Torki M, Zangeneh S, Habibian M (2014) Performance, egg quality traits, and serum metabolite concentrations of laying hens affected by dietary supplemental chromium picolinate and vitamin C under a heat-stress condition. Biol Trace Elem Res 157(2):120–129
Tórtora-Pérez JL (2010) The importance of selenium and the effects of its deficiency in animal health. Small Rumin Res 89(2–3):185–192
Trindade Neto MAD, Kobashigawa E, Namazu LB, Takeara P, Araújo LF, Albuquerque RD (2010) Lisina digestível e zinco orgânico para frangos de corte machos na fase de 22 a 42 dias de idade. Revista Brasileira de Zootecnia 39:2460–2470
Umar YM, Wang G, Sun W, Pei X, Liu L, Tao W, Xiao Z, Wang M, Huai M, Li L, Pelletier W (2020) Effects of inorganic trace minerals replaced by complexed glycinates on reproductive performance, blood profiles, and antioxidant status in broiler breeders. Poult Sci 99:2718–26 https://doi.org/10.1016/j.psj.2019.11.058
Utlu N, Celebi S, Yücel O (2007) The effects of natural zeolite supplementation to diet on serum element concentrations in laying hens. Revue de Medecine Veterinaire 158:598–602
Venglovska K, Gresakova L, Placha I, Ryzner M, Cobanova K (2014) Effects of feed supplementation with manganese from its different sources on performance and egg parameters of laying hens. Czeh J Anim Sci 59:147–155
Wang Y, Zhan X, Yuan D, Zhang X, Wu R (2011) Influence of dietary selenomethionine supplementation on performance and selenium status of broiler breeders and their subsequent progeny. Biol Trace Elem Res 143(3):1497–1507. https://doi.org/10.1007/s12011-011-8976-2
Wang L, Johnson EE, Shi HN, Walker WA, Wessling- Resnick M, Cherayil BJ (2008) Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol 181:2723–2731
Woods SL, Sobolewska S, Rose SP, Whiting IM, Blanchard A, Ionescu C, Pirgozliev V (2020) Effect of feeding different sources of selenium on growth performance and antioxidant status of broilers. Br Poult Sci 61(3):274–280
Woyengo TA, Nyachoti CM (2013) Review: Anti-nutritional effects of phytic acid in diets for pigs and poultry—current knowledge and directions for future research. Can J Anim Sci 93:9–21
Xiao JF, Zhang YN, Wu SG, Zhang HJ, Yue HY, Qi GH (2014) Manganese supplementation enhances the synthesis of glycosaminoglycan in eggshell membrane: a strategy to improve eggshell quality in laying hens. Poult Sci 93(2):380–388
Xie C, Elwan HA, Elnesr SS, Dong X, Feng J, Zou XT (2019) Effects of iron glycine chelate on laying performance, antioxidant activities, serum biochemical indices, iron concentrations and transferrin mRNA expression in laying hens. J Anim Physiol Anim Nutr 103(2):547–554
Xie C, Elwan HAM, Elnesr SS, Dong XY, Zou XT (2019) Effect of iron glycine chelate supplementation on egg quality and egg iron enrichment in laying hens. Poult Sci 98(12):7101–7109
Xu CL, Ji C, Ma Q, Hao K, Jin ZY, Li K (2006) Effects of a dried Bacillus subtilis culture on egg quality. Poult Sci 85:364–368
Yenice E, Mızrak C, Gültekin M, Atik Z, Tunca M (2015) Effects of organic and inorganic forms of manganese, zinc, copper, and chromium on bioavailability of these minerals and calcium in late-phase laying hens. Biol Trace Elem Res 167(2):300–307. https://doi.org/10.1007/s12011-015-0313-8
Yıldız AÖ, Cufadar Y, Olgun O (2011) Effects of dietary organic and inorganic manganese supplementation on performance, egg quality and bone mineralisation in laying hens. Revue de Medecine Veterinaire 162(10):482–488
Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B, Uddin MS, Aleya L, Abdel-Daim MM (2020) Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res 27(16):19151–68
Zarghi H, Hassanabadi A, Barzegar N (2023) Effect of organic and inorganic manganese supplementation on performance and eggshell quality in aged laying hens. Vet Med Sci 9(3):1256–1268
Zhang KK, Han MM, Dong YY, Miao ZQ, Zhang JZ, Song XY, Feng Y, Li HF, Zhang LH, Wei QY, Xu JP, Gu DC, JH, Li (2021) Low levels of organic compound trace elements improve the eggshell quality, antioxidant capacity, immune function, and mineral deposition of aged layinghens. Animal 15:100401. https://doi.org/10.1016/j.animal.2021.10040
Zhang LY, Lu L, Zhang LY, Luo XG (2016) The chemical characteristics of organic iron sources and their relative bioavailabilities for broilers fed a conventional corn–soybean meal diet. J Anim Sci 94:2378–2396
Zhang LY, Lu L, Zhang LY, Luo XG (2016) The chemical characteristics of organic iron sources and their relative bioavailabilities for broilers fed a conventional corn–soybean meal diet. J Anim Sci 94:2378–2396
Zhang X, Tian L, Zhai S, Lin Z, Yang H, Chen J, Zhu Y (2020) Effects of selenium-enriched yeast on performance, egg quality, antioxidant balance, and egg selenium content in laying ducks. Front Vet Sci 7:591
Zhang YN, Wang J, Zhang HJ, Wu SG, Qi GH (2017) Effect of dietary supplementation of organic or inorganic manganese on eggshell quality, ultrastructure, and components in laying hens. Poult Sci 96(7):2184–2193
Zhu Y, Chen P, Wan H, Wang Y, Hao P, Liu Y, Liu J (2018) Selenium-chromium (VI) interaction regulates the contents and correlations of trace elements in chicken brain and serum. Biol Trace Elem Res 181(1):154–163. https://doi.org/10.1007/s12011-017-1038-7
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all works conducted in the present study. All authors have drafted, reviewed, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
No ethical approval was required as this is a review article with no original research data.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elnesr, S.S., Mahmoud, B.Y., da Silva Pires, P.G. et al. Trace Minerals in Laying Hen Diets and Their Effects on Egg Quality. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04121-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04121-8