Abstract
Aflatoxin B1 (AFB1) is a plant-origin toxin that could induce oxidative stress in fish. The micromineral selenium (Se) possesses well-documented antioxidant properties. To assess the ameliorative effects of SeNPs (1 mg/kg fish feed) on oxidative stress induced by AFB1 (500 μg/kg fish feed), Nile tilapia (32.2±1.7 g body weight) were distributed randomly and even in six groups for 8-week feeding trial. Live enzymes, AST, ALT, and ALP levels were increased in the serum of fish fed AFB1-contaminated diet, and the addition of SeNPs could restore normal values compared to the control. The gene expression of antioxidant enzymes, superoxide dismutase (SOD) enzyme and catalase (CAT) enzyme, and DNA fragmentation were significantly increased in response to aflatoxin exposure, while dietary SeNPs could mitigate the generated oxidative stress. The innate immunity, serum antibacterial activity (SAA), oxidative burst activity (OBA), phagocytic activities (PA and PI), and gene expression of cytokines (interleukin (IL)-1β, heat shock protein70 (Hsp), and tumor necrosis factor (TNF)-α) revealed a status of immunosuppression in Nile tilapia fed on AFB1-contaminated diet. These findings showed that fish became more vulnerable to Streptococcus agalactiae infection with a high mortality rate while dietary SeNPs provided a high relative protection level (RPL). From the obtained findings, SeNPs could mitigate the oxidative stress induced by feeding the AFB1 diet and could boost the immunity of stressed Nile tilapia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia is currently among the most widely farmed fish species in the world, second only to carp, with production of over 6 million mt in 2019, valued at over 12 billion US$ [1]. Wherein Egypt, Nile tilapia fish (Oreochromis niloticus) is the most preferred cultured fish species by Egyptian consumers due to its high palatability and convenient price [2]. The high production of freshwater fish farms places Egypt in the second rank following China all over the world [3].
Additionally, high price fish meal is a common source of protein in most aquafeeds [4]; therefore, replacement of fish meal with cereal crops in aquatic diet formulation is necessary, and unfortunately, plant-based diets contain various antinutrients, such as phytate, which are chelated with trace minerals, rendering them unavailable [5, 6]. So, supplemental essential trace minerals are necessary for fish feeds at optimum levels [7]. Microminerals such as selenium (Se), manganese (Mn), zinc (Zn), copper (Cu), iodine (I), and iron (Fe) play different roles in the fish body, including the development and homeostasis of the skeletal system; composition of organic molecules such as proteins, lipids, and hormones; enhancement of enzymatic activities; and maintenance of the osmotic balance [6,7,8]. Several studies investigate the use of nanosized minerals to combat bacterial infection in Nile tilapia such as Se, titanium dioxide nanoparticles (anatase and rutile crystal) and zinc nanoparticles [9,10,11,12,13,14], and natural products such as Moringa oleifera [15], Nigella sativa oil [14], and silymarin [16]. The micromineral (Se) possesses an essential metabolic role saving the antioxidant status in the animal body [17, 18]. Meanwhile, Se is an essential micromineral in animal feed with a narrow therapeutic index against immunotoxicity [19]. Currently, several studies stated that nanometals could be used in fish feed to fulfill their dietary requirements, and SeNPs boost immune and antioxidant status along with high bioavailability and lower toxicity in Nile tilapia [2], common carp [20], and rainbow trout [21]. Recently, organic nanomaterials have been preferred, and Se-enriched yeast (Se-yeast) has become the main used form [22]. The nutritional requirements of selenium were estimated in different fish species: 1.06–2.06 mg/kg for Nile tilapia [23] and 0.73–1.19 mg/kg for gibel carp (Carassius auratus gibelio var. CAS III) [24].
Nile tilapia that received SeNPs at a level of 2 mg/kg fish feed showed significant enhancements in growth performance and survival rate [13]. Dietary selenium yeast at the rate of 3.3 mg/kg diet (2.36 mg selenomethionine and 0.94 mg organic selenium) for 60 days could protect Nile tilapia against chronic toxicity of glyphosate and/or malathion via mitigating the generated oxidative inflammation [11]. Naturally, selenium presents in organic forms as selenomethionine and selena-cysteine [25] and inorganic elemental state (Se0) as selenides (Se2−), selenates, or selenites [26]. Many factors affect the selenium transformation pH, amount of free oxygen, redox potential, and humidity [27].
Many authors suggested different dietary supplementation rates of Se, Se-enriched yeast 0.5–4 g/kg [11], animal feeds of 0.5 mg/kg [28], up to 0.2 mg/kg (1–1.1 mg/kg assessed dietary selenium) in juvenile gilthead seabream [29].
The extensive use of cereal ingredients in feed formulations may lead to an increase in the occurrence of aflatoxicosis, mainly with improper storage [30, 31]. Hence, Nile tilapia is by far the most cultivated fish species in Egypt and is highly AFB1 sensitive [32]. Feeding AFB1-contaminated feed resulted in generated oxidative stress, which led to immunotoxicity, inflammation reaction, and apoptosis in animals [33, 34].
Selenium is an antioxidant agent that induces the production of glutathione peroxidase (GPx) to counteract oxidative stress [35]. Se deficiency, fish became more vulnerable to microbial infection and sensitivity to AFB1-induced toxicity in the spleen, which is one of the lymphoid organs responsible for humoral and cellular immune responses [36, 37]. Detoxification of AFB1 by physical and chemical methods has several drawbacks, such as loss of some nutrients and decreased palatability. Moreover, the equipment required for the application of these techniques is too expensive for farmers [38].
So, this work investigates the oxidative stress in Nile tilapia that resulted from feeding on AFB1-contaminated diet. Also, the potential ameliorating role of nanoselenium SeNPs was evaluated against aflatoxin (AFB1) stress.
Materials and Methods
Fish Accommodation and Trial Design
Experimental Nile tilapia (Oreochromis niloticus), with an average weight of 32.2 ± 1.7 g, were purchased from a local private freshwater fish farm at Um-sin village in Kafrelsheikh, Egypt. Nile tilapia was transported to wet the laboratory of the Animal Health Research Institute. According to [39,40,41], fish were tranquilized at the farm with MS222 (SyncaineR, Syndel, Canada) at a dose of 40 mg/L. On arrival, the fish were disinfected with a bath of iodine (20 ppm/L), the trade name is Betadine® containing 5% povidone-iodine, the product was purchased from the local market and manufactured by Nile Company for Pharmaceuticals, Egypt, and then fish were stocked in fiberglass tank 1.5 × 1 m. Water quality was checked day after day.
In the wet laboratory, fish were acclimatized for 14 days at experimental conditions: water temperature, pH, and salinity were 27.5 ± 0.5 °C, 7.9 ± 0.1, and 0.48 ± 0.1 g/L, respectively. Day after day, only one-third of tank water was exchanged with clean un-chlorinated water to maintain constantly suitable water parameters.
Fish feed was offered at 0.9.00 a.m. and 03.30 p.m. at a rate of 5% b.w. fish per day, basal diet composition: crude protein 30%, digestible energy 4000 kcal/kg. The diet was purchased from the local market and manufactured by Aller Aqua® Egypt (Reg. no. 9782). Lot. no. 365622 https://www.aller-aqua.com/.
Fish were subdivided into five groups and fed on diets supplemented with SeNPs and AFB1 at a concentration of 1 mg/kg [2] and 500 μg/kg fish feed [37], respectively, following:
-
G1: Fish fed the basal diet are considered as a control group.
-
G2: Fish fed the basal diet supplied with SeNPs for 4 weeks.
-
G3: Fish fed an AFB1-contaminated diet for 4 weeks.
-
G4: Fish fed an AFB1-contaminated diet plus SeNPs for 4 weeks.
-
G5: Fish fed an AFB1-contaminated for 4 weeks then SeNPs with the basal diet for another 4 weeks.
-
G6: Fish fed an AFB1-contaminated diet for 4 weeks then SeNPs plus AFB1-contaminated diet for another 4 weeks.
Additive Sources and Preparation
Aflatoxin (AFB1) Preparation
Following the method of Abdelhamid and Mahmoud [42], corn pellets were fermented using Aspergillus parasiticus (NRRL 2999) which was grown in synthetic media, yeast extract-sucrose broth containing 2% yeast extract and 20% sucrose. The substrate was dispensed in conical flasks. The flasks were then autoclaved for 15 min at 121 °C, then cooled and inoculated with spore suspension and incubated for 9 days at 25–29 °C then AFB1 concentration was determined using quantitative thin layer chromatography TLC [43].
Preparation of SeNPs and Diet Incorporation
The SeNPs were manufactured following the methods described by Zommara [44] and Prokisch et al. [45] using lactic acid bacteria (LAB-Se, Lactomicrosel®). Briefly, SeNPs were manufactured using pure yogurt bacterial cultures containing Lactobacillus delbrueckii subsp. bulgaricus (NCAIM B 02206) and Streptococcus thermophilus (CNCM I-1670). The size of the obtained SeNPs was determined and photographed using a scanning electron microscope (SEM) (JSM-IT100, JEOL Co. Japan) photos of the cultured media after 72 h [46, 47]. The manufactured SeNPs were uniformly distributed in Milli-Q water (1 mg/mL) using an ultrasonic [48]. Thereafter, fish food (pellet form) was soaked and fully homogenated until paste formation. Then, the gelatine was added to feed past/Se NP mixture to improve feed consistency (Canal Aqua Cure, Egypt) and left to dry at room temperature then was evenly cut into small sizes.
Liver Enzymes
The levels of the liver enzymes in the serum of the experimental fish, aspartate amino transaminase (AST) and alanine amino transaminase (ALT), were colometerically detected to evaluate the effect of AFB1 and the ameliorative role of SeNPs according to the methods reported by Reitman and Frankel [49]. AST used aspartate and 2-oxoglutarate to obtain glutamate and oxalacetate and ALT used alanine and 2-oxoglutrate and obtain glutamate and pyruvate. The oxalacetate or pyruvate formed in the above reaction reacts with 2-4-dinitrophenyl hydrazine to form phenyl hydrazone. The end compound generates a color that could be measured at the wavelength of 546 nm. The color intensity is related to enzyme activity compared to a standard reference. Levels of alkaline phosphatase (ALP) activity were detected according to methods mentioned by Rec [50]. All kits and reagents were provided by Spectrum Diagnostic Co.
Innate Immunity
Serum Antibacterial Activity (SAA)
The activity of Nile tilapia serum was tested against bacterial in fish that received dietary-active-MOS and the control [51]; briefly, O. niloticus serum and a bacterial suspension of A. hydrophila (2 × 108 CFU) were combined in equal quantities (100 μL) and incubated at 25 °C for 1 h. Furthermore, the blank (control) was formed with sterile PBS instead of fish serum. A dilution at 1:10 with sterile PBS of 100 μL of the serum-bacterial mixture was placed on blood agar and placed in an incubator (27 °C/24 h). The colonies that grew on the tryptic soy agar were counted as viable bacteria.
Oxidative Burst Activity (OBA)
According to Anderson et al. [52], the OBA of the experimental fish heterophils was determined using a nitroblue tetrazolium assay briefly; within 15 min following the blood sample collection, a drop of heparinized fish blood was put on a coverslip then the coverslips were incubated in humid chambers for 30 min at room temperature (25 °C), and the neutrophils adhere to the coverslip, which was gently washed with PBS (pH 7.4). The coverslip was stained with NBT 0.2% solution. The stain was manufactured by Fluka Buchs, Switzerland. After 30 min of incubation, the dark blue-stained cells were considered positive and counted under a light microscope.
Phagocytosis Activity
Following the method of Kawahara et al. [53], heterophiles were isolated [54], then a 24-h-old of culture Candida albicans with a dose of 1 × 106 cells/mL, and heterophile count was adjusted at 2.5 × 106 viable cells/mL, and a mixture of 1 mL of the C. albicans suspension and 1 mL of collected heterophiles was incubated 27 °C/1 h with 5–10% CO2. The mixtures were smeared and stained with Giemsa. A minimum of 100 cells were counted in different fields under a microscope at 1000× magnification. The phagocytic assay PA and the phagocytic index (PI) were calculated using the following equations:
Antioxidant and Cytokine Gene Expressions
The impacts of intermittent dietary-active-MOS on the gene expression of immune-related cytokines; interleukin (IL)-1β, heat shock (Hsp)70, and tumor necrosis factor (TNF)-α, besides antioxidant enzymes; superoxide dismutase enzyme (SOD) and catalase (CAT) enzyme. At the end of the trial, three Nile tilapia were used to extract total RNA from the head kidney tissues using the Trizol reagent (iNtRON Biotechnology Inc., Korea) according to the manufacturer’s recommendations.
By Nanodrop D-1000 spectrophotometer, the harvested RNA was evaluated for quantity and quality, spectrophotometer manufactured by NanoDrop Technologies Inc., USA. The obtained cDNA was used as a template in the quantitative real-time PCR assay, and the housekeeping gene was β-actin due to its constitutive gene expression, and the expression was determined using the equation 2−ΔΔCT [55]. All primers are listed in Table 1.
DNA Fragmentation Assays
According to Perandones et al. [56], DNA fragmentation was determined in the experimental Nile tilapia. The principle is a bluish color that developed from the reaction of deoxyribose a sugar (derived from DNA) with perchloric acid and the diphenylamine reagent.
Bacterial Challenge
At the end of the feeding trial, ten Nile tilapia from each group were randomly chosen and injected via intraperitoneally (IP) route with LD50 (3 × 105 CFU) of S. agalactiae pathogenic strain (accession number OL471408) strain previously isolated and identified by Sherif et al. [57]. In addition, ten fish from the control group were injected with pure saline solution (0.65%) and were considered negative controls [58]. For 14 days, the injected Nile tilapia were kept under observation to calculate the mortality rate (MR %).
The relative protection levels (RLP) of SeNPs were evaluated according to Ruangpan et al. [59] as follows:
Statistics
The obtained data which concerning the impacts of AFB1 contamination and dietary SeNPs in Nile tilapia were analyzed for the significance of differences between the experimental groups at P ≤ 0.05 using one-way ANOVA by SPSS version 22 (SPSS Inc., IL, USA). The data are presented as the mean ± standard error.
Results
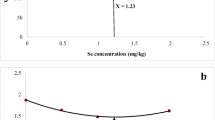
The Characterization of Biological SeNPs
In Fig. 1, the biological method was used to produce SeNPs with a size ranging between 100 and 200 nm. The final mixture contains lactic acid bacteria (L. bulgaricus).
Activity of Liver Enzymes in Serum of Nile Tilapia
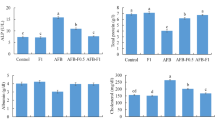
In Fig. 2, Nile tilapia tested with AFB1-contaminated diets and/or SeNPs, AST, ALT, and ALP levels were upregulated in the serum of fish fed AFB1 diet (G3), 123.5, 145.7, and 85.7, respectively (P ≤ 0.05), while fish received SeNPs with free diet after AFB1 diet for 4 weeks (G5) had significantly low levels 41.5, 32.2, and 27 U/L, respectively (P ≤ 0.05), compared to G3. Liver enzymes of fish (G4 and G6) that fed AFB1-contaminated diets and received SeNPs were decreased compared to G3.
Liver enzymes of the experimental Nile tilapia. Note: AST, aspartate amino transaminase; ALT, alanine amino transaminase; ALP, alkaline phosphatase. Fish groups (G) fed diets: G1, basal diet; G2, SeNP diet; G3, AFB1 diet; G4, AFB1 plus SeNP diet; G5, AFB1 diet then basal diet plus SeNPs; G6, AFB1 diet then AFB1 diet plus SeNPs. Different capital letters indicate significant difference at P ≤ 0.05
Innate Immunity of Nile Tilapia Exposed to AFB1 and Received SeNPs
In Table 2, significant alterations in SAA, OBA, and phagocytic activities (PA and PI) were observed in Nile tilapia fed AFB1 diet (G3), and aflatoxicosis resulted in immunosuppressed status. Supplementation with dietary SeNPs significantly (P ≤ 0.05) elevates the immune status (G2) even in fish that received AFB1 diets (G4 and G6).
Dietary SeNPs boost the antibacterial compound in fish serum SAA was significantly raised (45.20%) compared to the control 33.70% (P ≤ 0.05); meanwhile, a drastic decline was observed in groups exposed to AFB1 (G3, 14.10%) and SeNP supplementation could partially restore SAA (G5, 40.60%).
The ability of phagocytic cells to adhere to (OBA) and phagocyte (PA and PI) microbes was significantly restored after ceasing AFB1 exposure and adding dietary SeNPs (G5) (P ≤ 0.05).
Gene Expression of Antioxidant Enzymes in the Liver of Nile Tilapia Exposed to AFB1 and Received SeNPs
In Fig. 3, in Nile tilapia, gene expressions of SOD and CAT were significantly increased in response to aflatoxicosis (G3) 9.5- and 8.72-fold change, respectively (P ≤ 0.05) compared to the other groups; meanwhile, ceasing AFB1 diet and feeding dietary SeNPs (G5) could counteract the generated oxidative status and downregulate the gene expressions of antioxidant enzymes 2.1- and 1.8-fold change, respectively.
Gene expression antioxidant enzymes of the experimental Nile tilapia. Note: SOD, superoxide dismutase enzyme; CAT, catalase. Fish groups (G) fed diets: G1, basal diet; G2, SeNP diet; G3, AFB1 diet; G4, AFB1 plus SeNP diet; G5, AFB1 diet then basal diet plus SeNPs; G6, AFB1 diet then AFB1 diet plus SeNPs. Different capital letters indicate significant difference at P ≤ 0.05
Gene Expression of Cytokines in the Liver of Nile Tilapia Exposed to AFB1 and Received SeNPs
In Fig. 4, to study the impacts of AFB1 on the immune system, the gene expressions of IL-1β, Hsp70, and TNF-α were assessed in experimental Nile tilapia. Regardless of SeNP addition, fish received AFB1 diets were immunosuppressed as IL-1β, Hsp70, and TNF-α were significantly decreased (P ≤ 0.05), while Nile tilapia received dietary SeNPs were significantly immune enhanced (P ≤ 0.05).
Gene expression of cytokines of the experimental Nile tilapia. IL, interleukin; Hsp, heat shock protein; TNF, tumor necrosis factor. Fish groups (G) fed diets: G1, basal diet; G2, SeNP diet; G3, AFB1 diet; G4, AFB1 plus SeNP diet; G5, AFB1 diet then basal diet plus SeNPs; G6, AFB1 diet then AFB1 diet plus SeNPs. Different capital letters indicate significant difference at P ≤ 0.05
DNA Fragmentation of Liver Cells of Nile Tilapia Exposed to AFB1 and Received SeNPs
In Fig. 5, feeding an AFB1-contaminated diet increased DNA fragmentation (G3) reaching about 51.2% compared to the control 9.8%. The addition of SeNPs in Nile tilapia diets could ameliorate the withdrawals of aflatoxicosis and decrease DNA fragmentation to 12.42%, 24.5%, and 34.5% in G5, G4, and G6, respectively.
DNA fragmentation of the experimental Nile tilapia. IL, interleukin; Hsp, heat shock protein; TNF, tumor necrosis factor. Fish groups (G) fed diets: G1, basal diet; G2, SeNP diet; G3, AFB1 diet; G4, AFB1 plus SeNP diet; G5, AFB1 diet then basal diet plus SeNPs; G6, AFB1 diet then AFB1 diet plus SeNPs
Experimental Infection Test
In Table 3, Nile tilapia fed dietary SeNPs and experimentally infected with S. agalactiae had a low MR of 40% providing 33.3% of RLP. Fish feed contaminated diets supplemented with SeNPs had a similar MR% as the control ones about 60%, while the MR of fish that received SeNPs with free diet (G5) was similar to G2 at 40%. As RPL% was calculated, fish of G3 were considered the control positive to those fed contaminated diets and received SeNPs (G4, G5, and G6) providing 40%, 60%, and 40%, respectively.
Discussion
Aflatoxins adversely affected the aquaculture industry such as the cost of toxin elimination chelating materials and the loss in net fish production. These toxins induce oxidative inflammation by generating ROS production, causing cellular damage. Selenium is a well-known antioxidant agent that could be used as feed supplementation for aquatic animals.
In this study, the biologically manufactured SeNPs with 100- to 200-nm size were used in a feeding trial at a concentration of 1 mg/kg fish feed to boost the immunity of Nile tilapia and to mitigate oxidative stress which was induced by aflatoxicosis. Different dietary requirements were set for Se, with a maximum of 0.2 mg/kg in animal feed [60], between 0.18 and 0.38 mg/kg [61], 1.06 mg/kg and 2.06 mg/kg Se for Nile tilapia [23]. In this experiment, no deaths were recorded in the experimentally fed contaminated diet with AFB1 and/or SeNPs. Accordingly, biological-origin SeNPs were used safely in animal feed [20]. Similarly, SeNPs as its toxicity less by 3.6 times than selenomethionine [62]. In accordance, Gonçalves et al. [63] observed a high survival rate of 99.0–99.7% in Tra catfish (Pangasius hypophthalmus) after 12 weeks of exposure to AFB1 (0, 50, 100, and 250 μg/kg), whereas Nile tilapia fed with 2000 μg/kg of AFB1 had a survival rate of only 82.2% [64].
Before macroscopic changes took place, alterations in serum AST and ALT could be noticed after receiving dietary AFB1 in gibel carp [65] and Tra catfish [63]. In this study, Nile tilapia that fed AFB1-contaminated diets, AST, ALT, and ALP levels were upregulated in the serum at 123.5, 145.7, and 85.7 U/L, respectively. Similarly, AST and ALT were increased in the serum of Nile tilapia that received dietary AFB1 at a concentration of 20 and 100 μg/kg for 12 weeks [2]. These findings could be explained by the findings of [66] who stated that large amounts of toxins metabolized in the liver that damage the tissue and raise the enzyme activities. Other results found by Deng et al. [67]; ALT and AST levels did not alter in Nile tilapia fed AFB1-contaminated diet at a concentration of 1.641 μg/kg and in Gibel carps at a concentration up to 1000 μg/kg. On the contrary, some researchers found that fish fed dietary AFB1 at a concentration of 100 μg/kg fish feed did not affect such as Nile tilapia [64, 68] and South American catfish (Rhamdia quelen) [69]. These different findings related to AFB1 concentration, feeding period, and fish species.
No changes were recorded in serum AST, ALT, and ALP levels of the experimental fish that received dietary SeNPs. Similarly, Liu et al. [70] found that ALT and AST levels in the serum of grass carp (Ctenopharyngodon Idella) were significantly increased in fish fed with a high-fat diet and dietary SeNPs restored normal levels. Meanwhile, Neamat-Allah et al. [71] stated that ALT, AST, ALP, LDH, and creatinine in Nile tilapia were significantly increased in serum with dietary SeNPs. This could be due to the liver of fish being the site of Se metabolism and accumulation [72]. These authors use high dietary SeNPs and/or longer periods; in addition, the raise was only in numbers not in duplicates of the control level that could not considered clinical cases.
Aflatoxins are immunosuppressive substances that cause weakness the innate immunity [73]. Experimental Nile tilapia fed an AFB1-contaminated diet were immunosuppressed, with SAA, OBA, and phagocytic activities significantly declined. Accordingly, aflatoxin metabolites in the liver could bind with cellular macromolecules preventing their synthesis [74] and leading to a significant decline of serum proteins in sea bass (Dicentrarchus labrax) [75] and Nile tilapia [76]. Dietary-SeNPs enhanced the innate immunity of Nile tilapia. In accordance, Se is a part of selenoprotein that could increase serum protein production, so using SeNPs as a dietary supplementation could enhance non-specific immunity [77, 78]; in addition, they were less toxic to immune cells compared with organic and inorganic forms [79].
The gene expression of SOD and CAT were significantly downregulated in the experimental Nile tilapia fed AFB1 diet compared to the control fish. Similarly, the activities of CAT and GSH were decreased leading to a decline of antioxidant capacity in Nile tilapia that fed diets contaminated with AFB1 [64]. The study findings indicated that Se supplementation could ameliorate the generated oxidative stress resulting from AFB1. Glutathione peroxidase (GPx), CAT, SOD, myeloperoxidase (MPO), and lysozyme (LZM) in Nile tilapia were improved by dietary Se supplementation [80, 81]. In addition, oxidative stress could be significantly mitigated by using dietary SeNPs that stimulate the formation of glutathione [6, 82, 83].
From the obtained results, Nile tilapia that fed an AFB1-contaminated diet showed an upsurge of DNA fragmentation in hepatic tissue [84], and dietary SeNPs could counteract these withdrawals. Similarly, SeNPs overcome the adverse impacts of aflatoxicosis by increasing the synthesis of protein and DNA reducing the deaths of lymphocyte cells and DNA fragmentation [85], also suppressing the pro-apoptotic proteins leading to a decrease of oxidative damage in the liver [33], along with triggering the carotenoids and vitamin A remediation role [83].
The gene expressions of IL-1β, Hsp-70, and TNF-α of the experimental Nile tilapia were decreased after receiving AFB1-contaminated feed while dietary SeNPs could ameliorate such hazards. In accordance, feeding AFB1-contaminated diets resulted in heightened expression of IL-6, IFN-γ, and IL-10; impaired lymphocyte; and delayed cell-mediated immune response [86], while Se deficiency aggravated AFB1-induced immunotoxicity [87]. Similarly, dietary SeNPs (5 mg/kg) protected rainbow trout (Oncorhynchus mykiss) against oxidative stress by inducing the gene expression of Hsp70b, Hsp90α, and Hsp30, selenoproteins (Gpx1a, Gpx1b1, and Trx), and TGF-β as well as catalase activity [88].
The experimental Nile tilapia were experimentally infected with S. agalactiae; high MR% was recorded in fish that fed AFB1-contaminated feed while dietary SeNPs provided high RPL%. Similarly, survival rates of Nile tilapia were upsurged by dietary Se when they were challenged against S. agalactiae infection [81, 89], Aeromonas hydrophila [90, 91], and S. iniae [71]. In addition, antibacterial effects were increased by increasing Se concentration as 0.1–0.3 mg/kg exerting more activity than 1.5 mg/kg, this is due to the peptidoglycan layer destruction in the bacterial cell membrane [92] that kills the pathogen via cytoplasmic leakage [93]. Similarly, Nile tilapia that fed a diet supplemented with PSP-SeNPs (selenium nanoparticles coated with polysaccharide-protein) at a rate of 0.1–0.3 mg/kg could combat oxidative stress and experimental Streptococcus agalactiae infection [92]. Accordingly, with different forms of Se, Hassan et al. [94] found that organic selenium could ameliorate oxidative stress following the exposure to single exposure to malathion and glyphosate, as well as combat A. hydrophila infection.
Conclusion
From previous findings, SeNPs could partially ameliorate aflatoxicosis (AFB1) in Nile tilapia. Oxidative stress was the most prominent sign accompanied by AFB1 and demonstrated by increasing DNA fragmentation and gene expressions of antioxidant enzymes along with immunosuppression. The addition of SeNPs enhanced the antioxidant status, especially with a free AFB1 diet. Meanwhile, continuous feeding on contaminated diets with/without SeNP supplementation fish could not restore normal physiological parameters. Nile tilapia that received AFB1 diet became more vulnerable to bacterial infection while SeNP supplementation provided influential protection.
References
FAO (Food and Agriculture Organization of the United Nations) (2021) Global aquaculture production 1950–2019. http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en
Sherif AH, Gouda MY, Zommara MA, Abd El-Rahim AH, Mahrous KF, Salama ASS (2021a) Inhibitory effect of nano selenium on the recurrence of Aeromonas hydrophila bacteria in Cyprinus carpio. Egypt J Aquat Biol Fish 25(3):713–738. https://doi.org/10.21608/EJABF.2021.180901
FAO (Food and Agriculture Organization of the United Nations) (2012) The state of world fisheries and aquaculture. The role of capture fisheries in a global sustainable food production system: opportunities and challenges. FAO, Rome, pp 199–207
Rana KJ, Siriwardena S, Hasan MR (2009) Impact of rising feed ingredient prices on aquafeeds and aquaculture production (No. 541): IV–IX. FAO, Rome, p 63
Fontainhas-fernandes A, Gomes E, Reis-Henriques MA, Coimbra J (1999) Replacement of fish meal by plant proteins in the diet of Nile tilapia: digestibility and growth performance. Aquac int 7:57–67
El-Sayed AFM (2020) Nutrition and feeding. In: El-Sayed A-FM (ed) Tilapia culture, 2nd edn. Elsevier/Academic Press, London and Oxford, pp 135–172
Antony Jesu Prabhu P, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. Rev Aquac 8(2):172–219. https://doi.org/10.1111/raq.12090
Sherif AH, Abdelsalam M, Ali NG, Mahrous KF (2022b) Zinc oxide nanoparticles boost the immune responses in Oreochromis niloticus and improve disease resistance to Aeromonas hydrophila infection. Biol Trace Elem Res 201:927–936. https://doi.org/10.1007/s12011-022-03183-w
Sherif AH, Alsokary ET, Esam HA (2019) Assessment of titanium dioxide nanoparticle as treatment of Aeromonas hydrophila infection in Oreochromis niloticus. J Hell Vet Med Soc 70(3):1697–1706. https://doi.org/10.12681/jhvms.21796
Sherif AH, El-Sharawy MES, El-Samannoudy SI et al (2021c) The deleterious impacts of dietary titanium dioxide nanoparticles on the intestinal microbiota, antioxidant enzymes, diseases resistances and immune response of Nile tilapia. Aquacult Res 52(12):6699–6707. https://doi.org/10.1111/are.15539
Hassan MA, Hozien ST, Abdel Wahab MM, Hassan AM (2022) Ameliorative effect of selenium yeast supplementation on the physio-pathological impacts ofchronic exposure to glyphosate and or malathion in Oreochromis niloticus. BMC Vet Res 18(1):159
Sherif AH, Khalil RH, Tanekhy M, Sabry NM, Harfoush MA, Elnagar MA (2022c) Lactobacillus plantarum ameliorates the immunological impacts of titanium dioxide nanoparticles (rutile) in Oreochromis niloticus. Aquacult Res 53:3736–3747. https://doi.org/10.1111/are.15877
Sheikh S, Ghojaghi F, Ghelichi A, Jorjani S (2023) Dietary effects of selenium nanoparticles on growth performance, survival rate, chemical composition, and muscle bioaccumulation of Nile tilapia (Oreochromis niloticus). Biol Trace Elem Res:1–6. https://doi.org/10.1007/s12011-023-03836-4
Sherif AH, Elkasef M, Mahfouz ME, Kasem EA (2023d) Impacts of dietary zinc oxide nanoparticles on the growth and immunity of Nile tilapia could be ameliorated using Nigella sativa oil. J Trace Elem Med Biol 79:127265. https://doi.org/10.1016/j.jtemb.2023.127265
Sherif AH, Prince A, Adel Seida A, Saad Sharaf M, Eldessouki EA, Harfoush MA (2022e) Moringa oleifera mitigates oxytetracycline stress in Oreochromis niloticus. Aquacult Res 53(5):1790–1799. https://doi.org/10.1111/are.15707
Sherif AH, Toulan AE, El-kalamwi N et al (2023e) Silymarin enhances the response to oxytetracycline treatment in Oreochromis niloticus experimentally infected with Aeromonas hydrophila. Sci Rep 13:16235. https://doi.org/10.1038/s41598-023-43270-z
Biller-Takahashi JD, Takahashi LS, Mingatto FE, Urbinati EC (2015) The immune system is limited by oxidative stress: dietary selenium promotes optimal antioxidative status and greatest immune defense in pacu Piaractus mesopotamicus. Fish Shellfish Immunol 47(1):360–367. https://doi.org/10.1016/j.fsi.2015.09.022
Zhang Z, Li S, Jiang H, Liu B, Lv Z, Guo C, Zhang H (2017) Effects of selenium on apoptosis and abnormal amino acid metabolism induced by excess fatty acid in isolated rat hepatocytes. Mol Nutr Food Res 61(9):1700016. https://doi.org/10.1002/mnfr.201700016
Ungvari E, Monori I, Megyeri A, Csiki Z et al (2014) Protective effects of meat from lambs on selenium nanoparticle supplemented diet in a mouse model of polycyclic aromatic hydrocarbon-induced immunotoxicity. Food Chem Toxicol 64:298–306
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi HJAN (2017) Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquacult Nutr 23(3):611–617. https://doi.org/10.1111/anu.12428
Kohshahi AJ, Sourinejad I, Sarkheil M, Johari SA (2019) Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 45:793–804
Ferrari L, Cattaneo DM, Abbate R et al (2023) Advances in selenium supplementation: from selenium-enriched yeast to potential selenium-enriched insects, and selenium nanoparticles. Anim Nutr 14:193–203. https://doi.org/10.1016/j.aninu.2023.05.002
Lee S, Nambi RW, Won S, Katya K, Bai SC (2016) Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 464:153–158. https://doi.org/10.1016/j.aquaculture.2016.06.027
Zhu L, Han D, Zhu X et al (2017a) Dietary selenium requirement for on-growing gibel carp (Carassius auratus gibelio var. CAS III). Aquacult Res 48(6):2841–2851. https://doi.org/10.1111/are.13118
Kieliszek MJM (2019) Selenium–fascinating microelement, properties and sources in food. Molecules 24(7):1298
Thoennessen MJRPP (2013) Current status and future potential of nuclide discoveries. Rep Prog Phys 76(5):056301
Kieliszek M, Błażejak S (2016) Current knowledge on the importance of selenium in food for living organisms: a review. Molecules 21(5):609
EFSA (2012) Panel on Additives Products or Substances used in Animal, F. F. Scientific Opinion on safety and efficacy of selenium in the form of organic compounds produced by the selenium-enriched yeast Saccharomyces cerevisiae NCYC R646 (Selemax 1000/2000) as feed additive for all species. EFSA J 10:2778. https://doi.org/10.2903/j.efsa.2012.2778
Mechlaoui M et al (2019) Effects of different dietary selenium sources on growth performance, liver and muscle composition, antioxidant status, stress response and expression of related genes in gilthead seabream (Sparus aurata). Aquaculture 507:251–259. https://doi.org/10.1016/j.aquaculture
Rodrigues I, Naehrer K (2012) A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 4(9):663–675
Zychowski KE, Pohlenz C, Mays T, Romoser A et al (2013) The effect of NovaSil dietary supplementation on the growth and health performance of Nile tilapia (Oreochromis niloticus) fed aflatoxin-B1 contaminated feed. Aquaculture 376:117–123
Kenawy AM, El-Genaidy HM, Authman MM, Abdel-Wahab MA (2009) Pathological studies on effects of aflatoxin on Oreochromis niloticus with application of different trials of control. Egypt J Comp Path Clinic Path 22(1):175–193
Sun LH, Zhang NY, Zhu MK, Zhao L, Zhou JC, Qi DS (2015) Prevention of aflatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome P450 isozymes and up-regulation of 6 selenoprotein genes in chick liver. J Nutr 146(4):655–661
Zhu P, Zuo Z, Zheng Z, Wang F et al (2017b) Aflatoxin B1 affects apoptosis and expression of death receptor and endoplasmic reticulum molecules in chicken spleen. Oncotarget 8(59):99531
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590
Wang F, Shu G, Peng X, Fang J et al (2013) Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int J Environ Res Public Health 10(7):2834–2844
Sherif AH, Elshenawy AM, Attia AA, Salama SAA (2021b) Effect of Aflatoxin B1 on farmed Cyprinus carpio in conjunction with bacterial infection. Egypt J Aquat Biol Fish 25(2):465–485. https://doi.org/10.21608/EJABF.2021.164686
Patriarca A, Pinto VF (2017) Prevalence of mycotoxins in foods and decontamination. Curr Opin Food Sci 14:50–60
Eldessouki EA, Salama SSA, Mohamed R, Sherif AH (2023) Using nutraceutical to alleviate transportation stress in the Nile tilapia. Egypt J Aquat Biol Fish 27(1):413–429. https://doi.org/10.21608/ejabf.2023.287741
Sherif AH, Farag EA, Mahmoud AE (2023a) Temperature fluctuation alters immuno-antioxidant response and enhances the susceptibility of Oreochromis niloticus to Aeromonas hydrophila challenge. Aquac Int:1–14. https://doi.org/10.1007/s10499-023-01263-9
Sherif AH, Eldessouki EA, Sabry NM, Ali NG (2023b) The protective role of iodine and MS-222 against stress response and bacterial infections during Nile tilapia (Oreochromis niloticus) transportation. Aquac Int:401–416. https://doi.org/10.1007/s10499-022-00984-7
Abdelhamid AM, Mahmoud KI (1996) Elimination or adsorption of aflatoxin from poultry feedstuffs. In: Proc. Conf. Foodborne contamination & Egyptian’s Health. Mansoura Univ, pp 26–27
Eppley RM (1968) Screening method for zearalenone. Aflatoxin, and ochratoxin. J Assoc Off Anal Chem 51(1):74–78. https://doi.org/10.1093/jaoac/51.1.74
Zommara MA (2007) Production of organic selenium enriched yoghurt. J Agric Res Kafr El-Shaikh Univ, pp 31–820
Prokisch J, Széles É, Kovács B, Daróczy L, Zommara M (2008) Formation of metal selenium nanospheres in bacteria: is it a possible detoxification mechanism? Cereal Res Commun 36:947–950 https://www.jstor.org/stable/90002862
Eszenyi P, Sztrik A, Babka B, Prokisch J (2011) Production of Lactomicrosel® and nanosize (100-500 NM) selenium spheres by probiotic lactic acid bacteria. In: International Conference on Food Engineering and Biotechnology IPCBEE, vol 9, pp 858–862
Prokisch J, Sztrik A, Babka B, Eszenyi P, Pardi J, Mika Z, Zommara M (2011) Novel fermentation technology for production of selenium nanospheres (Lactomicrosel®) and its testing for feed and food applications. In: 2nd International conference on selenium in the environment and human health china-singapore suzhou industrial park, Suzhou, China
Lammel T, Sturve J (2018) Assessment of titanium dioxide nanoparticle toxicity in the rainbow trout (Onchorynchus mykiss) liver and gill cell lines RTL-W1 and RTgill-W1 under particular consideration of nanoparticle stability and interference with fluorometric assays. NanoImpact 11:1–19. https://doi.org/10.1016/j.impact.2018.01.001
Reitman S, Frankel S (1957) Determination of AST and ALT in serum. Am J Clin Pathol 28:56–68
Rec GSCC (1972) Colorimetric method for serum alkaline phosphatase determination. J Clin Chem Clin Biochem 10(2):182
Kajita Y, Sakai M, Atsuta S, Kobayashi M (1990) The immunomodulatory effects of levamisole on rainbow trout, Oncorhynchus mykiss. Fish Pathol 25(2):93–98
Anderson DP, Moritomo T, de Grooth R (1992) Neutrophil, glass-adherent, nitroblue tetrazolium assay gives early indication of immunization effectiveness in rainbow trout. Vet Immunol Immunopathol 30(4):419–429. https://doi.org/10.1016/0165-2427(92)90110-C
Kawahara E, Ueda T, Nomura S (1991) In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol 26(4):213–214
Faulmann E, Cuchens MA, Lobb CJ, Miller NW, Clem LW (1983) An effective culture system for studying in vitro mitogenic responses of channel catfish lymphocytes. Trans Am Fish Soc 112(5):673–679. https://doi.org/10.1577/1548-8659
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Perandones CE, Illera,VA, Peckham D, Stunz LL, Ashman RF (1993) Regulation of apoptosis in vitro in mature murine spleen T cells. J Immun J Immun Balt 151(7): 3521-3529.
Sherif AH, Abdellatif JI, Elsiefy MM, Gouda MY, Mahmoud AE (2022a) Occurrence of infectious Streptococcus agalactiae in the farmed Nile tilapia. Egypt J Aquat Biol Fish 26(3):403–432. https://doi.org/10.21608/ejabf.2022.243162
Boijink CD, Brandao DA, Vargas AC, Costa MM, Renosto AV (2001) Inoculation of bacterial suspension of Plesiomonas shigelloides in jundiá, Rhamdia quelen (Teleostei: Pimelodidae) (teleostei: pimelodidae). Cienc Rural 31:497–501. https://doi.org/10.1590/S0103-84782001000300023
Ruangpan L, Kitao T, Yoshida T (1986) Protective efficacy ofAeromonas hydrophila vaccines in Nile tilapia. Vet Immunol Immunopathol 12(1-4):345–350. https://doi.org/10.1016/0165-2427(86)90139-X
Sele V, Ørnsrud R, Sloth JJ, Berntssen MH, Amlund H (2018) Selenium and selenium species in feeds and muscle tissue of Atlantic salmon. J Trace Elem Med Biol 47:124–133. https://doi.org/10.1016/j.jtemb.2018.02.005
NRC (2011) Nutrient requirements of fish. National Academies Press, 2101, Constitution Avenue, NW Washington, D.C. (20418)
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42(10):1524–1533. https://doi.org/10.1016/j.freeradbiomed.2007.02.013
Gonçalves RA, Do Cam T, Tri NN, Santos GA, Encarnação P, Hung LT (2018) Aflatoxin B 1 (AFB 1) reduces growth performance, physiological response, and disease resistance in Tra catfish (Pangasius hypophthalmus). Aquacu Int 26:921–936
Naiel MA, Ismael NE, Shehata SA (2019) Ameliorative effect of diets supplemented with rosemary (Rosmarinus officinalis) on aflatoxin B1 toxicity in terms of the performance, liver histopathology, immunity and antioxidant activity of Nile Tilapia (Oreochromis niloticus). Aquaculture 511:734264
Huang Y, Han D, Zhu X, Yang Y, Jin J, Chen Y, Xie S (2011) Response and recovery of gibel carp from subchronic oral administration of aflatoxin B1. Aquaculture 319(1-2):89–97
Thrall MA, Baker DC, Campbell TW et al (2007) Hematologia e bioquímica clínica veterinária. Editora Roca. Hematologia e Bioquimica Clinica Veterinaria Roca, Sao Paulo, p 582
Deng SX, Tian LX, Liu FJ et al (2010) Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus× O. aureus) during long-term dietary exposure. Aquaculture 307(3-4):233–240
Abd El-Baki SM, Nowar MS, Hassona EA, Bassuny SM, Shehata SA (2002) Clays in animal nutrition: 10-detoxification of aflatoxin B1 by tafla clay in rabbit feeds. In: 3rd Sci. Con. on Rabbit Production in Hot Climates, pp 8–11
Anater A, Araújo CM, Rocha DC, Ostrensky A, Engracia Filho JR, Ribeiro DR, Pimpao CT (2020) Evaluation of growth performance, hematological, biochemical and histopathological parameters of Rhamdia quelen fed with a feed artificially contaminated with aflatoxin B1. Aquac Rep 17:100326
Liu G, Yu H, Wang C et al (2021) Nano-selenium supplements in high-fat diets relieve hepatopancreas injury and improve survival of grass carp Ctenopharyngodon Idella by reducing lipid deposition. Aquaculture 538:736580
Neamat-Allah AN, Mahmoud EA, Abd El Hakim Y (2019) Efficacy of dietary Nano-selenium on growth, immune response, antioxidant, transcriptomic profile and resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae infection. Fish Shellfish Immunol 94:280–287
Hodson PV, Spry DJ, Blunt BR (1980) Effects on rainbow trout (Salmo gairdneri) of a chronic exposure to waterborne selenium. Can J Fish Aquat Sci 37(2):233–240
Flores-flores ME, Lizarraga E, de Cerain AL, González-Peñas E (2015) Presence of mycotoxins in animal milk: a review. Food Control 53:163–176. https://doi.org/10.1016/j.foodcont.2015.01.020
Cagauan AG, Tayaban RH, Somga JR, Bartolome RM (2004) Effect of aflatoxin-contaminated feeds in Nile tilapia (Oreochromis niloticus L.). In Abstract of the 6th international symposium on tilapia in aquaculture (ISTA 6) section: health management and diseases Manila. Philippines 12:172–178
El-Sayed YS, Khalil RH (2009) Toxicity, biochemical effects and residue of aflatoxin B1 in marine water-reared sea bass (Dicentrarchus labrax L.). Food Chem Toxicol 47(7):1606–1609. https://doi.org/10.1016/j.fct.2009.04.008
Hessein A, Marakby H, Abd Rahamn G, Ayyat M (2014) Aflatoxin B 1 toxicity and its reduction by using coumarine and vitamin E in Nile tilapia. Zagazig J Agric Res 73:83
Mansour ATE, Goda AA, Omar EA, Khalil HS, Esteban MÁ (2017) Dietary supplementation of organic selenium improves growth, survival, antioxidant and immune status of meagre, Argyrosomus regius, juveniles. Fish Shellfish Immunol 68:516–524. https://doi.org/10.1016/j.fsi.2017.07.060
Jingyuan H, Yan L, Wenjing P et al (2020) Dietary selenium enhances the growth and anti-oxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol 101:115–125. https://doi.org/10.1016/j.fsi.2020.03.041
Nikapitiya C, Dananjaya SHS, De Silva BCJ, Heo GJ, Oh C, De Zoysa M, Lee J (2018) Chitosan nanoparticles: a positive immune response modulator as display in zebrafish larvae against Aeromonas hydrophila infection. Shellfish Immunol 76:240–246. https://doi.org/10.1016/j.fsi.2018.03.010
Rathore SS, Murthy HS, Girisha SK et al (2021) Supplementation of nano-selenium in fish diet: Impact on selenium assimilation and immune-regulated selenoproteome expression in monosex Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol Part-C: Toxicol Pharmacol 240:108907. https://doi.org/10.1016/j.cbpc.2020.108907
Wangkahart E, Bruneel B, Chantiratikul A et al (2022) Optimum dietary sources and levels of selenium improve growth, antioxidant status, and disease resistance: re-evaluation in a farmed fish species, Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 121:172–182
Salem SS, Fouda A (2021) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199(1):344–370. https://doi.org/10.1007/s12011-020-02138-3
Hamed GMS, Selim MA (2021) Protective effect of nano-selenium against experimentally induced toxicity by aflatoxin B1 (AFB1) on the gingiva and periodontal ligament of albino rats (histological and immunohistochemical study). Egypt Dent J 67(1):357–366
Abdel-Aziem SH, Hassan AM, Abdel-Wahhab MA (2011) Dietary supplementation with whey protein and ginseng extract counteracts oxidative stress and DNA damage in rats fed an aflatoxin-contaminated diet. Mutat Res Genet Toxicol Environ Mutagen 723(1):65–71
Bhattacharjee S (2016) DLS and zeta potential–what they are and what they are not? J Control Release 235:337–351. https://doi.org/10.1016/j.jconrel.2016.06.017
Pierron A, Alassane-Kpembi I, Oswald IP (2016) Impact of mycotoxin on immune response and consequences for pig health. Anim Nutr 2(2):63–68
Zhao L, Feng Y, Deng J et al (2019) Selenium deficiency aggravates aflatoxin B1–induced immunotoxicity in chick spleen by regulating 6 selenoprotein genes and redox/inflammation/apoptotic signaling. J Nutr 149(6):894–901
Li L, Liu Z, Quan J, Sun J, Lu J, Zhao G (2023) Dietary nano-selenium alleviates heat stress-induced intestinal damage through affecting intestinal antioxidant capacity and microbiota in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 133:108537. https://doi.org/10.1016/j.fsi.2023.108537
Sherif AH, AbuLeila RH (2022) Prevalence of some pathogenic bacteria in caged- Nile tilapia (Oreochromis Niloticus) and their possible treatment. Jordan. Aust J Biol Sci 15(2):239–247. https://doi.org/10.54319/jjbs/150211
Ibrahim D, Neamat-Allah AN, Ibrahim SM et al (2021) Dual effect of selenium loaded chitosan nanoparticles on growth, antioxidant, immune related genes expression, transcriptomics modulation of caspase 1, cytochrome P450 and heat shock protein and Aeromonas hydrophila resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 110:91–99. https://doi.org/10.1016/j.fsi.2021.01.003
Sherif AH, Kassab AS (2023) Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol 23(1):80. https://doi.org/10.1186/s12866-023-02827-8
Ni J, Ren L, Ma Y, Xiong H, Jian W (2023) Selenium nanoparticles coated with polysaccharide-protein complexes from abalone viscera improve growth and enhance resistance to diseases and hypoxic stress in juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 134:108624. https://doi.org/10.1016/j.fsi.2023.108624
Muthu S, Raju V, Gopal VB, Gunasekaran A, Narayan KS, Malairaj S, Lakshmikanthan M, Duraisamy N, Krishnan K, Perumal P (2019) A rapid synthesis and antibacterial property of selenium nanoparticles using egg white lysozyme as a stabilizing agent. SN Appl Sci 1:1–9. https://doi.org/10.1007/s42452-019-1509-x
Hassan MA, Hozien ST, Abdel Wahab MM, Hassan AM (2022) Risk assessment of glyphosate and malathion pollution and their potential impact on Oreochromis niloticus: role of organic selenium supplementation. Sci Rep 12(1):9992
Availability of Data and Materials
Data are available on request from the corresponding author.
Code Availability
Not applicable
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Funding Open access funding provided by the Science, Technology & Innovation Funding Authority (STDF) in cooperation with the Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this work. All authors analyze and interpreted the data regarding gene expression and enzymes. All authors performed the experimental study and were major contributors to writing the manuscript. All authors read, reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The above described methodology was approved by the Ethics Committee at the Animal Health Research Institute and European Union directive 2010/63UE, and all methods were carried out in accordance with relevant guidelines and regulations. This study is reported in accordance with ARRIVE guidelines (https:// arriv eguid elines. org). This paper does not contain any studies with human participants by any of the authors. No specific permissions were required for access to the artificial pond in wet laboratory Animal Health Research Institute, Kafrelsheikh, Egypt. The field studies did not involve endangered or protected species.
Consent to Participate
Not applicable
Consent to Publish
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(JPG 142 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sherif, A.H., Zommara, M.A. Selenium Nanoparticles Ameliorate Adverse Impacts of Aflatoxin in Nile Tilapia with Special Reference to Streptococcus agalactiae Infection. Biol Trace Elem Res (2023). https://doi.org/10.1007/s12011-023-04031-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-023-04031-1