Abstract
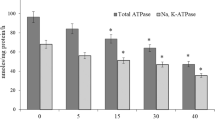
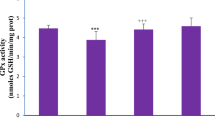
Copper (Cu) is a heavy metal that is widely used in industries and is also an essential micronutrient for living beings. However, excess Cu is toxic and human exposure to high levels of this metal results in numerous adverse health effects. We have investigated the effect of oral administration of copper chloride (CuCl2), a Cu(II) compound, on various parameters of oxidative stress, cellular metabolism, and DNA integrity in the rat kidney. This was done to delineate the molecular mechanism of Cu(II) toxicity. Adult male rats were randomly divided into five groups. Animals in four CuCl2-treated groups were separately administered single acute oral dose of CuCl2 at 5, 15, 30, and 40 mg/kg body weight. Animals in the fifth group were not given CuCl2 and served as the control. All rats were sacrificed 24 h after the dose of CuCl2 and their kidneys removed. CuCl2 administration led to significant alterations in enzymatic and non-enzymatic parameters of oxidative stress. It changed the activities of metabolic and membrane bound enzymes and also decreased the activities of brush border membrane enzymes. CuCl2 treatment dose-dependently enhanced DNA damage and DNA–protein crosslinking in renal cells, when compared to the control group. The administration of CuCl2 also resulted in marked morphological changes in the kidney, with more prominent alterations at higher doses of CuCl2. These results clearly show that CuCl2 impairs the antioxidant defense system resulting in oxidative damage to the kidney.






Similar content being viewed by others
Data Availability
The data analyzed during the study are available from the corresponding author on reasonable request.
References
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Itoh S, Ozumi K, Kim HW et al (2009) Novel mechanism for regulation of extracellular SOD transcription and activity by copper: role of antioxidant-1. Free Radic Biol Med 46:95–104
Tokar EJ, Boyd WA, Freedman JH, Waalkes MP (2013) Toxic effects of metals. Casarett and Doull’s toxicology: the basic science of poisons (Klaassen CD, Ed), McGraw Hill, New York 981–1030
Kahlson MA, Dixon SJ (2022) Copper-induced cell death. Sci 375:1231–1232. https://doi.org/10.1126/science.abo3959
Carvalho JA, Boavida L, Ferreira R et al (2021) Copper-induced haemolytic anaemia. Eur J Case Rep Intern Med 8:002785. https://doi.org/10.12890/2021_002785
Lorincz MT (2018) Wilson disease and related copper disorders. Handb Clin Neurol 147:279–292. https://doi.org/10.1016/B978-0-444-63233-3.00018-X
Lucena-Valera A, Perez-Palacios D, Muñoz-Hernandez R et al (2021) Wilson’s disease: revisiting an old friend. World J Hepatol 13:634–649. https://doi.org/10.4254/wjh.v13.i6.634
Attri S, Sharma N, Jahagirdar S et al (2006) Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr Res 59:593–597. https://doi.org/10.1203/01.pdr.0000203098.77573.39
Arnal N, Cristalli DO, de Alaniz MJT, Marra CA (2010) Clinical utility of copper, ceruloplasmin, and metallothionein plasma determinations in human neurodegenerative patients and their first-degree relatives. Brain Res 1319:118–130. https://doi.org/10.1016/j.brainres.2009.11.085
Singh SK, Balendra V, Obaid AA et al (2022) Copper-mediated β-amyloid toxicity and its chelation therapy in Alzheimer’s disease. Metallomics 14:mfac018. https://doi.org/10.1093/mtomcs/mfac018
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163. https://doi.org/10.1016/s0300-483x(03)00159-8
Tsang T, Davis CI, Brady DC (2021) Copper biology. Curr Biol 31:R421–R427. https://doi.org/10.1016/j.cub.2021.03.054
Letelier ME, Sánchez-Jofré S, Peredo-Silva L et al (2010) Mechanisms underlying iron and copper ions toxicity in biological systems: pro-oxidant activity and protein-binding effects. Chem Biol Interact 188:220–227. https://doi.org/10.1016/j.cbi.2010.06.013
Hayashi M, Kuge T, Endoh D et al (2000) Hepatic copper accumulation induces DNA strand breaks in the liver cells of Long-Evans Cinnamon strain rats. Biochem Biophys Res Commun 276:174–178. https://doi.org/10.1006/bbrc.2000.3454
Chen J, Jiang Y, Shi H et al (2020) The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Arch 472:1415–1429. https://doi.org/10.1007/s00424-020-02412-2
Letelier ME, Lepe AM, Faúndez M et al (2005) Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem Biol Interact 151:71–82. https://doi.org/10.1016/j.cbi.2004.12.004
Butler-Dawson J, James KA, Krisher L et al (2022) Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J Expo Sci Environ Epidemiol 32:461–471. https://doi.org/10.1038/s41370-021-00292-x
Lentini P, Zanoli L, Granata A et al (2017) Kidney and heavy metals - the role of environmental exposure (review). Mol Med Rep 15:3413–3419. https://doi.org/10.3892/mmr.2017.6389
Zhuang XH, Mo Y, Jiang XY, Chen SM (2008) Analysis of renal impairment in children with Wilson’s disease. World J Pediatr 4:102–105. https://doi.org/10.1007/s12519-008-0019-5
Chanpong A, Dhawan A (2022) Wilson disease in children and young adults - state of the art. Saudi J Gastroenterol 28:21–31. https://doi.org/10.4103/sjg.sjg_501_21
Pocino M, Malavé I, Baute L (1990) Zinc administration restores the impaired immune response observed in mice receiving excess copper by oral route. Immunopharmacol Immunotoxicol 12:697–713. https://doi.org/10.3109/08923979009019685
Markevičius A, Dringelienė A (2004) Comparison of lead and copper exposure effect on immune cells in mice. Acta Medica Lituanica 11:14–18
Ji X, Mo Y, Li H et al (2021) Gender-dependent reproductive toxicity of copper metal-organic frameworks and attenuation by surface modification. Nanoscale 13:7389–7402. https://doi.org/10.1039/d1nr01008e
Babaei H, Kheirandish R, Ebrahimi L (2012) The effects of copper toxicity on histopathological and morphometrical changes of the rat testes. Asian Pac J Trop Biomed 2:S1615–S1619. https://doi.org/10.1016/S2221-1691(12)60463-8
Zhang CH, Wang Y, Sun QQ et al (2018) Copper nanoparticles show obvious in vitro and in vivo reproductive toxicity via ERK mediated signaling pathway in female mice. Int J Biol Sci 14:1834–1844. https://doi.org/10.7150/ijbs.27640
Kalita J, Kumar V, Misra UK, Bora HK (2020) Movement disorder in copper toxicity rat model: role of inflammation and apoptosis in the corpus striatum. Neurotox Res 37:904–912. https://doi.org/10.1007/s12640-019-00140-9
Ren X, Xu Y, Zhang Y et al (2020) Comparative accumulation and transcriptomic analysis of juvenile Marsupenaeus japonicus under cadmium or copper exposure. Chemosphere 249:126157. https://doi.org/10.1016/j.chemosphere.2020.126157
Zamberlan DC, Halmenschelager PT, Silva LFO, da Rocha JBT (2020) Copper decreases associative learning and memory in Drosophila melanogaster. Sci Total Environ 710:135306. https://doi.org/10.1016/j.scitotenv.2019.135306
Liao J, Yang F, Yu W et al (2020) Copper induces energy metabolic dysfunction and AMPK-mTOR pathway-mediated autophagy in kidney of broiler chickens. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2020.111366
Tian F, Xiao Y, Li X et al (2015) Protective effects of Lactobacillus plantarum CCFM8246 against copper toxicity in mice. PLoS One. https://doi.org/10.1371/journal.pone.0143318
Kumar V, Kalita J, Bora HK, Misra UK (2016) Temporal kinetics of organ damage in copper toxicity: a histopathological correlation in rat model. Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2016.09.025
Wan F, Zhong G, Ning Z et al (2020) Long-term exposure to copper induces autophagy and apoptosis through oxidative stress in rat kidneys. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2019.110158
Husak VV, Mosiichuk NM, Kubrak OI et al (2018) Acute exposure to copper induces variable intensity of oxidative stress in goldfish tissues. Fish Physiol Biochem. https://doi.org/10.1007/s10695-018-0473-5
Khundmiri SJ, Asghar M, Khan F et al (1997) Effect of reversible and irreversible ischemia on marker enzymes of BBM from renal cortical PT subpopulations. Am J Physiol Renal Physiol 273:F849–F856
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Kempson SA, Kim JK, Northrup TE et al (1979) Alkaline phosphatase in adaptation to low dietary phosphate intake. Am J Physiol Endocrinol Metab 237:E465
Glossmann H, Neville DM (1972) Gamma-glutamyltransferase in kidney brush border membranes. FEBS Lett 19:340–344. https://doi.org/10.1016/0014-5793(72)80075-9
Goldmann DR, Schlesinger H, Segal S (1976) Isolation and characterization of the brush border fraction from newborn rat renal proximal tubule cells. Biochim Biophys Acta 419:251–260
Bergmeyer H (1974) Enzymatic assay of maltase. Methods of enzymatic analysis, 2nd edn. Academic Press, New York, pp 459–460
Beutler E (1984) Red cell metabolism: a manual of biochemical methods. Grune & Stratton, Orlando, FL
Habeeb AF (1972) Reaction of protein sulfhydryl groups with Ellman’s reagent. Meth Enzymol 25:457–464. https://doi.org/10.1016/S0076-6879(72)25041-8
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Meth Enzymol 186:464–478
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Meth Enzymol 52:302–310
Gay C, Gebicki JM (2000) A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem 284:217–220. https://doi.org/10.1006/abio.2000.4696
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Carlberg I, Mannervik B (1985) Glutathione reductase. Meth Enzymol 113:484–490
Tamura T, Stadtman TC (1996) A new selenoprotein from human lung adenocarcinoma cells: purification, properties, and thioredoxin reductase activity. Proc Natl Acad Sci USA 93:1006–1011
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Meth Enzymol 105:114–121
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Mishra K, Ojha H, Chaudhury NK (2012) Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chem 130:1036–1043. https://doi.org/10.1016/j.foodchem.2011.07.127
Khundmiri SJ, Asghar M, Khan F et al (2004) Effect of ischemia and reperfusion on enzymes of carbohydrate metabolism in rat kidney. J Nephrol 17:377–383
Crane RK, Sols A (1953) The association of hexokinase with particulate fractions of brain and other tissue homogenates. J Biol Chem 203:273–292
Shonk CE, Boxer GE (1964) Enzyme patterns in human tissues. I. Methods for the determination of glycolytic enzymes. Cancer Res 24:709–721
Bonting SL, Simon KA, Hawkins NM (1961) Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative distribution in several tissues of the cat. Arch Biochem Biophys 95:416–423
Levinson SA, Macfate RP (1969) Clinical laboratory diagnosis, 7th ed. In: Clinical laboratory diagnosis, 7th ed. Lea and Febiger, Philadelphia, p 413
Burton K (1956) A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 62:315
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Zhitkovich A, Costa M (1992) A simple, sensitive assay to detect DNA-protein crosslinks in intact cells and in vivo. Carcinogenesis 13:1485–1489
Culling CFA (2013) Handbook of histopathological and histochemical techniques: including museum techniques. Butterworth-Heinemann
Radi R (2004) Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 101:4003–4008
Fahmy HM, Ebrahim NM, Gaber MH (2020) In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J Trace Elem Med Biol 60:126481. https://doi.org/10.1016/j.jtemb.2020.126481
Liu J, Wang Y, Zhao H et al (2020) Arsenic (III) or/and copper (II) exposure induce immunotoxicity through trigger oxidative stress, inflammation and immune imbalance in the bursa of chicken. Ecotoxicol Environ Saf 190:110127. https://doi.org/10.1016/j.ecoenv.2019.110127
Kumar V, Kalita J, Bora HK, Misra UK (2016) Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol Appl Pharmacol 293:37–43. https://doi.org/10.1016/j.taap.2016.01.007
Escobar JA, Rubio MA, Lissi EA (1996) Sod and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic Biol Med 20:285–290
Pigeolet E, Corbisier P, Houbion A et al (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51:283–297
Yaribeygi H, Farrokhi FR, Rezaee R, Sahebkar A (2018) Oxidative stress induces renal failure: a review of possible molecular pathways. J Cell Biochem 119:2990–2998. https://doi.org/10.1002/jcb.26450
Legouis D, Faivre A, Cippà PE, de Seigneux S (2022) Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrol Dial Transplant 37:1417–1425. https://doi.org/10.1093/ndt/gfaa302
Halliwell B, Aruoma OI (1991) DNA damage by oxygen-derived species Its mechanism and measurement in mammalian systems. FEBS Lett 281:9–19
Marnett LJ (2002) Oxy radicals, lipid peroxidation and DNA damage. Toxicology 181:219–222
Kwok ML, Hu XL, Meng Q, Chan KM (2020) Whole-transcriptome sequencing (RNA-seq) analyses of the zebrafish liver cell line, ZFL, after acute exposure to Cu2+ ions. Metallomics. https://doi.org/10.1039/d0mt00005a
Guecheva T, Henriques JA, Erdtmann B (2001) Genotoxic effects of copper sulphate in freshwater planarian in vivo, studied with the single-cell gel test (comet assay). Mutat Res 497:19–27
Jiang WD, Liu Y, Jiang J et al (2015) Copper exposure induces toxicity to the antioxidant system via the destruction of Nrf2/ARE signaling and caspase-3-regulated DNA damage in fish muscle: amelioration by myo-inositol. Aquat Toxicol 159:245–255. https://doi.org/10.1016/j.aquatox.2014.12.020
Guo H, Wang Y, Cui H et al (2022) Copper induces spleen damage through modulation of oxidative stress, apoptosis, DNA damage, and inflammation. Biol Trace Elem Res 200:669–677. https://doi.org/10.1007/s12011-021-02672-8
Dhanraj P, Venter C, Bester MJ, Oberholzer HM (2020) Induction of hepatic portal fibrosis, mitochondria damage, and extracellular vesicle formation in Sprague-Dawley rats exposed to copper, manganese, and mercury, alone and in combination. Ultrastruct Pathol:1–11. https://doi.org/10.1080/01913123.2020.1731638
Huang C, Shi Y, Zhou C et al (2021) Effects of subchronic copper poisoning on cecal histology and its microflora in chickens. Front Microbiol 12:739577. https://doi.org/10.3389/fmicb.2021.739577
Gherghina M-E, Peride I, Tiglis M et al (2022) Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci 23:3188. https://doi.org/10.3390/ijms23063188
Krata N, Zagożdżon R, Foroncewicz B, Mucha K (2018) Oxidative stress in kidney diseases: the cause or the consequence? Arch Immunol Ther Exp (Warsz) 66:211–220. https://doi.org/10.1007/s00005-017-0496-0
Acknowledgements
The authors would like to thank Samra Hasan, Amin Arif and Samreen Salam for their help in the experiments. We are indebted for the funding received by the Department of Biochemistry from University Grants Commission and Department of Science and Technology, New Delhi.
Author information
Authors and Affiliations
Contributions
Study conception, data analysis, and visualization were performed by NH, AAK, and RM. NH, SNA, and HA contributed to material preparations and lab experiments. RM designed the study. The first draft of the manuscript was written by NH and finalized by RM. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All animal procedures were approved by the Institutional Animal Ethics Committee of the Department of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University (Registration number: 714/GO/Re/S/02/CPCSEA). All experiments were done according to the guidelines and as recommended by the Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Husain, N., Ali, S.N., Arif, H. et al. Oral Administration of Copper Chloride Damages DNA, Lowers Antioxidant Defense, Alters Metabolic Status, and Inhibits Membrane Bound Enzymes in Rat Kidney. Biol Trace Elem Res 201, 3367–3380 (2023). https://doi.org/10.1007/s12011-022-03406-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03406-0