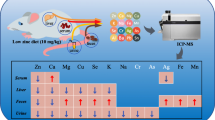
Abstract
Zinc (Zn) is an important trace element in the human body, and Zn deficiency affects the Zn content of major tissues. Marginal Zn deficiency is more common than severe Zn deficiency in humans. The objective of the present study was to compare the content and distribution of Zn and the change in the copper (Cu)–Zn superoxide dismutase (SOD) and metallothionein (MT) levels of soft tissues. Mice were fed with 30 mg/kg (control) or 10 mg/kg (marginally Zn-deficient, MZD) Zn diet for 35 days. We observed that only the Zn contents of serum, bones, and muscles in the control group were higher than those in the MZD group. Autometallography (AMG) was used as a method for staining Zn ions, and the semi-quantitative result indicated that the AMG products of the liver, duodenum, heart, lung, testes, and epididymis in the control group were higher than those in the MZD group. Furthermore, the contents of MT and the activities of Cu–Zn SOD in the testes, brain, duodenum, and liver were higher in the control group than those in the MZD group. However, the AMG products and the activities of Cu–Zn SOD of the kidney in the MZD group were more/higher than those in the control group. These results indicated that a change in the total Zn content of soft tissues may be not obvious and insensitive, and thus, more attention should be given to the distribution and localization of Zn ions. The functional indicators, MT and Cu–Zn SOD, are suitable biomarkers for evaluating zinc nutritional status. The brain, testes, duodenum, and liver are susceptive organs to Zn deficiency.






Similar content being viewed by others
References
Baltaci AK, Yuce K, Mogulkoc R (2018) Zinc metabolism and metallothioneins. Biol Trace Elem Res 1831:22–31. https://doi.org/10.1007/s12011-017-1119-7
Ugarte M, Osborne NN (2014) Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics 62:189–200. https://doi.org/10.1039/c3mt00291h
Prasad AS (2013) Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr 42:176–190. https://doi.org/10.3945/an.112.003210
Omu AE, Al-Azemi MK, Al-Maghrebi M, Mathew CT, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Memon A (2015) Molecular basis for the effects of zinc deficiency on spermatogenesis: An experimental study in the Sprague-dawley rat model. Indian J Urol 311:57–64. https://doi.org/10.4103/0970-1591.139570
Chen Y, Yang J, Wang Y, Yang M, Guo M (2020) Zinc Deficiency promotes testicular cell apoptosis in mice. Biol Trace Elem Res 1951:142–149. https://doi.org/10.1007/s12011-019-01821-4
Nuttall JR, Supasai S, Kha J, Vaeth BM, Mackenzie GG, Adamo AM, Oteiza PI (2015) Gestational marginal zinc deficiency impaired fetal neural progenitor cell proliferation by disrupting the ERK1/2 signaling pathway. J Nutr Biochem 2611:1116–1123. https://doi.org/10.1016/j.jnutbio.2015.05.007
McCormick NH, King J, Krebs N, Soybel DI, Kelleher SL (2015) Redistribution of tissue zinc pools during lactation and dyshomeostasis during marginal zinc deficiency in mice. J Trace Elem Med Biol 29:170–175. https://doi.org/10.1016/j.jtemb.2014.06.002
Jinno N, Nagata M, Takahashi T (2014) Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biol Trace Elem Res 1581:65–72. https://doi.org/10.1007/s12011-014-9901-2
Xu R, Chen MY, Liang W, Chen Y, Guo MY (2021) Zinc deficiency aggravation of ROS and inflammatory injury leading to renal fibrosis in mice. Biol Trace Elem Res 1992:622–632. https://doi.org/10.1007/s12011-020-02184-x
Cao JW, Duan SY, Zhang HX, Chen Y, Guo M (2020) Zinc deficiency promoted fibrosis via ROS and TIMP/MMPs in the myocardium of mice. Biol Trace Elem Res 1961:145–152. https://doi.org/10.1007/s12011-019-01902-4
Doboszewska U, Sowa-Kucma M, Mlyniec K, Pochwat B, Holuj M, Ostachowicz B, Pilc A, Nowak G, Szewczyk B (2015) Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog Neuropsychopharmacol Biol Psychiatry 56:254–263. https://doi.org/10.1016/j.pnpbp.2014.09.013
Romualdo GR, Goto RL, Henrique Fernandes AA, Cogliati B, Barbisan LF (2016) Dietary zinc deficiency predisposes mice to the development of preneoplastic lesions in chemically-induced hepatocarcinogenesis. Food Chem Toxicol 96:280–289. https://doi.org/10.1016/j.fct.2016.08.020
Zieminska E, Ruszczynska A, Augustyniak J, Toczylowska B, Lazarewicz JW (2021) Zinc and copper brain levels and expression of neurotransmitter receptors in two rat ASD models. Front Mol Neurosci 14:656740. https://doi.org/10.3389/fnmol.2021.656740
Staniek H (2019) The combined effects of Cr(III) propionate complex supplementation and iron excess on copper and zinc status in rats. J Trace Elem Med Biol 53:49–54. https://doi.org/10.1016/j.jtemb.2019.01.011
Kheirouri S, Alizadeh M (2014) Decreased serum and mucosa immunoglobulin A levels in vitamin A and zinc-deficient mice. Cent Eur J Immunol 392:165–169. https://doi.org/10.5114/ceji.2014.43716
Moran VH, Skinner AL, Medina MW, Patel S, Dykes F, Souverein OW, Dullemeijer C, Lowe NM (2012) The relationship between zinc intake and serum/plasma zinc concentration in pregnant and lactating women: a systematic review with dose-response meta-analyses. J Trace Elem Med Biol 262–3:74–79. https://doi.org/10.1016/j.jtemb.2012.04.003
Sirisena D, Gayashani Sandamalika WM, Neranjan Tharuka MD, Madusanka RK, Jeong JB, Lee J (2021) A copper-zinc-superoxide dismutase (CuZnSOD) from redlip mullet, Liza haematocheila: insights to its structural characteristics, immune responses, antioxidant activity, and potent antibacterial properties. Dev Comp Immunol 123:104165. https://doi.org/10.1016/j.dci.2021.104165
Tarhan C, Pekmez M, Karaer S, Arda N, Sarikaya AT (2007) The effect of superoxide dismutase deficiency on zinc toxicity in Schizosaccharomyces pombe. J Basic Microbiol 47:506–512. https://doi.org/10.1002/jobm.200700220
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection 14:1505–1517. https://doi.org/10.1089/ars.2010.3576
Sun JY, Jing MY, Weng XY, Fu LJ, Xu ZR, Zi NT, Wang JF (2004) Effects of dietary zinc levels on the activities of enzymes, weights of organs, and the concentrations of zinc and copper in growing rats. Biol Trace Elem Res 1072:153–165
Huang D, Hu Q, Fang S, Feng J (2016) Dosage effect of zinc glycine chelate on zinc metabolism and gene expression of zinc transporter in intestinal segments on rat. Biol Trace Elem Res 1712:363–370. https://doi.org/10.1007/s12011-015-0535-9
Kotani M, Kim KH, Ishizaki N, Funaba M, Matsui T (2013) Magnesium and calcium deficiencies additively increase zinc concentrations and metallothionein expression in the rat liver. Br J Nutr 1093:425–432. https://doi.org/10.1017/S0007114512001195
Calvo J, Jung H, Meloni G (2017) Copper metallothioneins. IUBMB Life 694:236–245. https://doi.org/10.1002/iub.1618
Kang YJ, Jiang Y, Saari JT (2007) Changes in copper and zinc status and response to dietary copper deficiency in metallothionein-overexpressing transgenic mouse heart. J Nutr Biochem 1811:714–718. https://doi.org/10.1016/j.jnutbio.2006.10.009
Brugger D, Windisch WM (2017) Short-term subclinical zinc deficiency in weaned piglets affects cardiac redox metabolism and zinc concentration. J Nutr 1474:521–527. https://doi.org/10.3945/jn.116.240804
Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A, Malavolta M (2011) Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenomic approach. Biogerontology 125:455–465. https://doi.org/10.1007/s10522-011-9337-4
Wang X, Wang ZY, Gao HL, Danscher G, Huang L (2006) Localization of ZnT7 and zinc ions in mouse retina–immunohistochemistry and selenium autometallography. Brain Res Bull 711–3:91–96. https://doi.org/10.1016/j.brainresbull.2006.08.002
Zhang LH, Wang X, Stoltenberg M, Danscher G, Huang L, Wang ZY (2008) Abundant expression of zinc transporters in the amyloid plaques of Alzheimer’s disease brain. Brain Res Bull 771:55–60. https://doi.org/10.1016/j.brainresbull.2008.03.014
Zhong M-L, Guo C, Chi Z-H, Shan Z-Y, Teng W-P and Wang Z-Y (2013) Distribution of zinc and zinc transporters in the mouse ovarian follicles and corpus luteum. Histology and Histopathology :1517–1527. https://doi.org/10.14670/HH-28.1517
Goldschmidt J, Wanger T, Engelhorn A, Friedrich H, Happel M, Ilango A, Engelmann M, Stuermer IW, Ohl FW, Scheich H (2010) High-resolution mapping of neuronal activity using the lipophilic thallium chelate complex TlDDC: protocol and validation of the method. Neuroimage 491:303–315. https://doi.org/10.1016/j.neuroimage.2009.08.012
Yokokawa H, Fukuda H, Saita M, Miyagami T, Takahashi Y, Hisaoka T, Naito T (2020) Serum zinc concentrations and characteristics of zinc deficiency/marginal deficiency among Japanese subjects. J Gen Fam Med 216:248–255. https://doi.org/10.1002/jgf2.377
Iwaya H, Kashiwaya M, Shinoki A, Lee JS, Hayashi K, Hara H, Ishizuka S (2011) Marginal zinc deficiency exacerbates experimental colitis induced by dextran sulfate sodium in rats. J Nutr 1416:1077–1082. https://doi.org/10.3945/jn.111.138180
Adamo AM, Liu X, Mathieu P, Nuttall JR, Supasai S, Oteiza PI (2019) Early developmental marginal zinc deficiency affects neurogenesis decreasing neuronal number and altering neuronal specification in the adult rat brain. Front Cell Neurosci 13:62. https://doi.org/10.3389/fncel.2019.00062
Croxford TP, McCormick NH, Kelleher SL (2011) Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr 1413:359–365. https://doi.org/10.3945/jn.110.131318
Gupta S, Brazier AKM, Lowe NM (2020) Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet 335:624–643. https://doi.org/10.1111/jhn.12791
Ozeki I, Arakawa T, Suii H, Tatsumi R, Yamaguchi M, Nakajima T, Kuwata Y, Toyota J (2020) Zinc deficiency in patients with chronic liver disease in Japan. Hepatol Res 503:396–401. https://doi.org/10.1111/hepr.13465
Maruyama Y, Nakashima A, Fukui A, Yokoo T (2021) Zinc deficiency: its prevalence and relationship to renal function in Japan. Clin Exp Nephrol 257:771–778. https://doi.org/10.1007/s10157-021-02046-3
Jing MY, Sun JY, Wang JF (2008) The effect of peripheral administration of zinc on food intake in rats fed Zn-adequate or Zn-deficient diets. Biol Trace Elem Res 1242:144–156. https://doi.org/10.1007/s12011-008-8132-9
Kambe T, Hashimoto A, Fujimoto S (2014) Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci 7117:3281–3295. https://doi.org/10.1007/s00018-014-1617-0
Kumari D, Nair N, Bedwal RS (2011) Effect of dietary zinc deficiency on testes of Wistar rats: morphometric and cell quantification studies. J Trace Elem Med Biol 251:47–53. https://doi.org/10.1016/j.jtemb.2010.11.002
Madej D, Pietruszka B, Kaluza J (2021) The effect of iron and/or zinc diet supplementation and termination of this practice on the antioxidant status of the reproductive tissues and sperm viability in rats. J Trace Elem Med Biol 64:126689. https://doi.org/10.1016/j.jtemb.2020.126689
Tubek S (2007) Zinc supplementation or regulation of its homeostasis: advantages and threats. Biol Trace Elem Res 1191:1–9. https://doi.org/10.1007/s12011-007-0043-7
Maret W (2015) Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 72:202–211. https://doi.org/10.1039/c4mt00230j
Danscher G, Stoltenberg M (2006) Silver enhancement of quantum dots resulting from (1) metabolism of toxic metals in animals and humans, (2) in vivo, in vitro and immersion created zinc-sulphur/zinc-selenium nanocrystals, (3) metal ions liberated from metal implants and particles. Prog Histochem Cytochem 412:57–139. https://doi.org/10.1016/j.proghi.2006.06.001
Davis S R and Cousin R J (2000) Metallothionein expression in animals: a physiological perspective on function. American society for nutritional sciences : 1085-1088. https://doi.org/10.1093/jn/130.5.1085.
K E J, J.Q C, F G J and P R D (1996) Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. Am Inst Nutr 1782-1790. https://doi.org/10.1093/jn/126.7.1782
Liu C, He X, Hong X, Kang F, Chen S, Wang Q, Chen X, Hu D, Sun Q (2014) Suppression of placental metallothionein 1 and zinc transporter 1 mRNA expressions contributes to fetal heart malformations caused by maternal zinc deficiency. Cardiovasc Toxicol 144:329–338. https://doi.org/10.1007/s12012-014-9256-0
Szczurek E I, Bjornsson C S and Taylor C G (2001) Dietary zinc deficiency and repletion modulate metallothionein immunolocalization and concentration in small intestine and liver of rats1,2. Nutr Metabol 2132-2138. https://doi.org/10.1093/jn/131.8.2132
Beckman JS, Estévez AG, Crow JP, Barbeito L (2001) Superoxide dismutase and the death of motoneurons in ALS. A Trends Guide to Neurodegenerative Disease and Repair 24:S15–S20. https://doi.org/10.1016/s0166-2236(00)01981-0
Ermilova IP, Ermilov VB, Levy M, Ho E, Pereira C, Beckman JS (2005) Protection by dietary zinc in ALS mutant G93A SOD transgenic mice. Neurosci Lett 3791:42–46. https://doi.org/10.1016/j.neulet.2004.12.045
Yin LL, Zhang Y, Guo DM, An K, Yin MS, Cui X (2013) Effects of zinc on interleukins and antioxidant enzyme values in psoriasis-induced mice. Biol Trace Elem Res 1553:411–415. https://doi.org/10.1007/s12011-013-9799-0
Deng B, Zhou X, Wu J, Long C, Yao Y, Peng H, Wan D, Wu X (2017) Effects of dietary supplementation with tribasic zinc sulfate or zinc sulfate on growth performance, zinc content and expression of zinc transporters in young pigs. Anim Sci J 8810:1556–1560. https://doi.org/10.1111/asj.12788
Cao Y, Li YS, Li ZJ, Wang F, Li CM (2015) Dietary zinc may attenuate heat-induced testicular oxidative stress in mice via up-regulation of Cu-Zn SOD. Genet Mol Res 144:16616–16626. https://doi.org/10.4238/2015.December.11.9
Gowanlock DW, Mahan DC, Jolliff JS, GM H, (2015) Evaluating the influence of National Research Council levels of copper, iron, manganese, and zinc using organic (Bioplex) minerals on resulting tissue mineral concentrations, metallothionein, and liver antioxidant enzymes in grower-finisher swine diets. J Anim Sci 93(3):1149–1156. https://doi.org/10.2527/jas2014-8173
Mattei D, Pietrobelli A (2019) Micronutrients and brain development. Curr Nutr Rep 82:99–107. https://doi.org/10.1007/s13668-019-0268-z
Elgazar V, Razanov V, Stoltenberg M, Hershfinkel M, Huleihel M, Nitzan YB, Lunenfeld E, Sekler I, Silverman WF (2005) Zinc-regulating proteins, ZnT-1, and metallothionein I/II are present in different cell populations in the mouse testis. J Histochem Cytochem 537:905–912. https://doi.org/10.1369/jhc.4A6482.2005
Hennigar SR, Olson CI, Kelley AM, McClung JP (2021) Slc39a4 in the small intestine predicts zinc absorption and utilization: a comprehensive analysis of zinc transporter expression in response to diets of varied zinc content in young mice. J Nutr Biochem 101:108927. https://doi.org/10.1016/j.jnutbio.2021.108927
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 579:399–411. https://doi.org/10.1016/s0753-3322(03)00081-7
Acknowledgements
Thanks to all members of Professor Wang CH’s lab at the School of Public Health, Wuhan University for their generous help. We would like to thank the animal facilities of Wuhan University Animal Experiment Center for the maintenance of the mouse population.
Funding
This study was supported by grants from Angel Nutritech Nutrition Fund (Grant NO. AF2019004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Peng, C., Zhang, Y. et al. Characteristics of Zn Content and Localization, Cu–Zn SOD, and MT Levels in the Tissues of Marginally Zn-Deficient Mice. Biol Trace Elem Res 201, 262–271 (2023). https://doi.org/10.1007/s12011-022-03119-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03119-4