Abstract
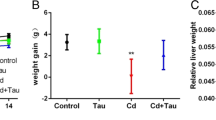
The toxic effects of cadmium (Cd) on hepatic parameters are widely described in the literature. Experimental models often make use of the intraperitoneal route (i.p.) because it is easier to apply, while in the oral route, Cd poisoning in humans is best represented by allowing the metal to pass through the digestive system and be absorbed into the bloodstream. Thus, this study investigated the Cd exposure impact on the liver, by comparing both i.p. and oral routes, both in single dose, in addition to the oral route in fractional doses. Swiss adult male mice received CdCl2 1.5 mg/kg i.p., 30 mg/kg oral single dose, and 4.28 mg/kg oral route in fractional doses for 7 consecutive days. Cd bioaccumulation was observed in all animals exposed to Cd. Hepatic concentrations of Ca and Fe increased only in the fractionated oral route. Liver activities of SOD and CAT increased only by oral single dose. GST decreased in all forms of oral administration, while MDA decreased only in i.p. route. Liver weight and HSI increased in the i.p. route, while organ volume increased in all forms of oral administration, and liver density increased in all animals exposed to Cd. In hepatic histomorphometry, the changes were more evident in oral administration, mainly in exposure to metal in a single dose. Thus, the subacute administration of Cd in different routes of administration leads to different changes in liver poisoning.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Sarkar A, Ravindran G, Krishnamurthy V (2013) A brief review on the effect of cadmium toxicity: from cellular to organ level. Int J Bio-Technology Res 3:2249–6858
de Angelis C, Galdiero M, Pivonello C et al (2017) The environment and male reproduction: the effect of cadmium exposure on reproductive functions and its implication in fertility. Reprod Toxicol 73:105–127. https://doi.org/10.1016/j.reprotox.2017.07.021
Fernandes GW, Goulart FF, Ranieri BD et al (2016) Deep into the mud: ecological and socio-economic impacts of the dam breach in Mariana, Brazil. Nat Conserv 14:35–45. https://doi.org/10.1016/j.ncon.2016.10.003
Cionek VM, Alves GHZ, Tófoli RM et al (2019) Brazil in the mud again: lessons not learned from Mariana dam collapse. Biodivers Conserv 28:1935–1938. https://doi.org/10.1007/s10531-019-01762-3
Lima AT, Bastos FA, Teubner FJ et al (2020) Strengths and weaknesses of a hybrid post-disaster management approach: the Doce River (Brazil) mine-tailing dam burst. Environ Manage. https://doi.org/10.1007/s00267-020-01279-4
ATSDR (2012) Toxicological profile for cadmium. Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services, Atlanta
Gao P, Liu S, Ye W et al (2015) Assessment on the occupational exposure of urban public bus drivers to bioaccessible trace metals through resuspended fraction of settled bus dust. Sci Total Environ 508:37–45. https://doi.org/10.1016/j.scitotenv.2014.11.067
Coelho DG, Marinato CS, Matos LP et al (2020) Evaluation of heavy metals in soil and tissues of economic-interest plants grown in sites affected by the Fundão dam failure in Mariana, Brazil. Integr Environ Assess Manag. https://doi.org/10.1002/ieam.4253
Bonecker ACT, Castro MS d, Costa PG et al (2019) Larval fish assemblages of the coastal area affected by the tailings of the collapsed dam in southeast Brazil. Reg Stud Mar Sci 32:100848. https://doi.org/10.1016/j.rsma.2019.100848
Akhgari M, Abdollahi M, Kebryaeezadeh A et al (2003) Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum Exp Toxicol 22:205–211. https://doi.org/10.1191/0960327103ht346oa
Salińska A, Wlostowski T, Zambrzycka E (2012) Effect of dietary cadmium and/or lead on histopathological changes in the kidneys and liver of bank voles Myodes glareolus kept in different group densities. Ecotoxicology 21:2235–2243. https://doi.org/10.1007/s10646-012-0979-z
Djuric A, Begic A, Gobeljic B et al (2015) Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium. Food Chem Toxicol 86:25–33. https://doi.org/10.1016/j.fct.2015.09.004
Cupertino M do C, Novaes RD, Santos EC et al (2017) Cadmium-induced testicular damage is associated with mineral imbalance, increased antioxidant enzymes activity and protein oxidation in rats. Life Sci 175:23–30. https://doi.org/10.1016/j.lfs.2017.03.007
Arafa MH, Mohammad NS, Atteia HH (2014) Fenugreek seed powder mitigates cadmium-induced testicular damage and hepatotoxicity in male rats. Exp Toxicol Pathol 66:293–300. https://doi.org/10.1016/j.etp.2014.04.001
Cupertino MC, Costa KLC, Santos DCM et al (2013) Long-lasting morphofunctional remodelling of liver parenchyma and stroma after a single exposure to low and moderate doses of cadmium in rats. Int J Exp Pathol 94:343–351. https://doi.org/10.1111/iep.12046
Rana K, Verma Y, Rana SVS (2020) Possible mechanisms of liver injury induced by cadmium sulfide nanoparticles in rat. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02128-5
Saedi S, Jafarzadeh Shirazi MR, Totonchi M et al (2020) Effect of prepubertal exposure to CdCl2 on the liver, hematological, and biochemical parameters in female rats; an experimental study. Biol Trace Elem Res 194:472–481. https://doi.org/10.1007/s12011-019-01800-9
de Souza Predes F, da Silva Diamante MA, Foglio MA et al (2014) Hepatoprotective effect of Arctium lappa root extract on cadmium toxicity in adult wistar rats. Biol Trace Elem Res 160:250–257. https://doi.org/10.1007/s12011-014-0040-6
Talcott MR, Akers W, Marini RP (2015) Chapter 25 - Techniques of Experimentation. In: Anderson LC, Otto G, Pritchett-Corning KR et al (eds) Laboratory animal medicine, 3rd edn, third edit. Elsevier Inc., pp 1201–1262
Zhu L, Duan P, Hu X et al (2019) Exposure to cadmium and mono-(2-ethylhexyl) phthalate induce biochemical changes in rat liver, spleen, lung and kidney as determined by attenuated total reflection-Fourier transform infrared spectroscopy. J Appl Toxicol 39:783–797. https://doi.org/10.1002/jat.3767
Mouro VGS, Martins ALP, Silva J et al (2019) Subacute testicular toxicity to cadmium exposure intraperitoneally and orally. Oxid Med Cell Longev 2019:1–14. https://doi.org/10.1155/2019/3429635
Mouro VGS, Siman VA, da Silva J et al (2019) Cadmium-induced testicular toxicity in mice: subacute and subchronic route-dependent effects. Biol Trace Elem Res. https://doi.org/10.1007/s12011-019-01731-5
Matović V, Dukić-Ćosić D, Buha A, Bulat Z (2013) Route, dose and duration of exposure to cadmium-relevance to oxidative stress induction. In: Bogaert L, Coppens N (eds) Peroxidases: biochemical characteristics, functions and potential applications. Nova Science Publishers, Inc., pp 159–175
Matović V, Buha A, Bulat Z et al (2012) Route-dependent effects of cadmium/cadmium and magnesium acute treatment on parameters of oxidative stress in rat liver. Food Chem Toxicol 50:552–557. https://doi.org/10.1016/j.fct.2011.12.035
Goering PL, Waalkes MP, Klaassen CD (1995) Toxicology of cadmium. In: Goyer RA, Cherian MG (eds) Toxicology of Metals: Biochemical Aspects. Springer Berlin Heidelberg, Berlin, pp 189–214
Oh S-H, Lee B-H, Lim S-C (2004) Cadmium induces apoptotic cell death in WI 38 cells via caspase-dependent Bid cleavage and calpain-mediated mitochondrial Bax cleavage by Bcl-2-independent pathway. Biochem Pharmacol 68:1845–1855. https://doi.org/10.1016/j.bcp.2004.06.021
Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118:182–190. https://doi.org/10.1289/ehp.0901234
de Souza Predes F, Diamante MAS, Dolder H (2010) Testis response to low doses of cadmium in Wistar rats. Int J Exp Pathol 91:125–131. https://doi.org/10.1111/j.1365-2613.2009.00692.x
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137–138
Novaes RD, Mouro VGS, Gonçalves RV et al (2018) Aluminum: a potentially toxic metal with dose-dependent effects on cardiac bioaccumulation, mineral distribution, DNA oxidation and microstructural remodeling. Environ Pollut 242:814–826. https://doi.org/10.1016/j.envpol.2018.07.034
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137–138
Kunifuji Y, Nakamura T, Takasugi M (1987) Influence of cadmium on the distribution of the essential trace elements zinc and copper in the liver and kidneys of rats. Biol Trace Elem Res 14:237–248. https://doi.org/10.1007/BF02795690
Müller L, Stacey NH (1989) Subclinical response to cadmium in liver cells. Biol Trace Elem Res 21:317–323. https://doi.org/10.1007/BF02917270
Abarikwu SO, Iserhienrhien BO, Badejo TA (2013) Rutin- and Selenium-attenuated cadmium-induced testicular pathophysiology in rats. Hum Exp Toxicol 32:395–406. https://doi.org/10.1177/0960327112472995
Abarikwu SO, Oruitemeka S, Uwadileke IA et al (2018) Oral administration of cadmium depletes intratesticular and epididymal iron levels and inhibits lipid peroxidation in the testis and epididymis of adult rats. J Trace Elem Med Biol 48:213–223. https://doi.org/10.1016/j.jtemb.2018.04.011
Cupertino MC, Novaes RD, Santos EC et al (2017) Differential susceptibility of germ and leydig cells to cadmium-mediated toxicity: impact on testis structure, adiponectin levels, and steroidogenesis. Oxid Med Cell Longev 2017. https://doi.org/10.1155/2017/3405089
Djordjevic VR, Wallace DR, Schweitzer A et al (2019) Environmental cadmium exposure and pancreatic cancer: evidence from case control, animal and in vitro studies. Environ Int 128:353–361. https://doi.org/10.1016/j.envint.2019.04.048
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117(285–297):50. https://doi.org/10.1016/S0092-8674(04)00343-5
Ryu DY, Lee SJ, Park DW, Choi BS, Klaassen CD, Park JD (2004) Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol Lett 152:19–25. https://doi.org/10.1016/j.toxlet.2004.03.015
El-Sokkary GH, Nafady AA, Shabash EH (2010) Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol Environ Saf 73:456–463. https://doi.org/10.1016/j.ecoenv.2009.09.014
Clapham DE (2007) Calcium Signaling. Cell 131:1047–1058. https://doi.org/10.1016/j.cell.2007.11.028
Boucherie S, Decaens C, Verbavatz J-M et al (2013) Cadmium disorganises the scaffolding of gap and tight junction proteins in the hepatic cell line WIF B9. Biol Cell 105:561–575. https://doi.org/10.1111/boc.201200092
Hu W, Jones PD, Upham BL et al (2002) Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci 68:429–436. https://doi.org/10.1093/toxsci/68.2.429
Han F, Yan S, Shi YX (2013) Single-prolonged stress induces endoplasmic reticulum - dependent apoptosis in the hippocampus in a rat model of post-traumatic stress disorder. PLoS One 8. https://doi.org/10.1371/journal.pone.0069340
Liu N, Huang H, Liu S et al (2014) Calcium channel blocker verapamil accelerates gambogic acid-induced cytotoxicity via enhancing proteasome inhibition and ROS generation. Toxicol Vitr 28:419–425. https://doi.org/10.1016/j.tiv.2013.12.008
Zou H, Liu X, Han T et al (2015) Alpha-lipoic acid protects against cadmium-induced hepatotoxicity via calcium signalling and gap junctional intercellular communication in rat hepatocytes. J Toxicol Sci 40:469–477. https://doi.org/10.2131/jts.40.469
Liao Y, Cao H, Xia B et al (2017) Changes in trace element contents and morphology in bones of duck exposed to molybdenum or/and cadmium. Biol Trace Elem Res 175:449–457. https://doi.org/10.1007/s12011-016-0778-0
Xu S, Pi H, Chen Y et al (2013) Cadmium induced Drp1-dependent mitochondrial fragmentation by disturbing calcium homeostasis in its hepatotoxicity. Cell Death Dis 4:1–10. https://doi.org/10.1038/cddis.2013.7
Casalino E, Sblano C, Landriscina C (1997) Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 346:171–179. https://doi.org/10.1006/abbi.1997.0197
Djukić-Ćosić D, Ćurčić Jovanović M, Plamenac Bulat Z et al (2008) Relation between lipid peroxidation and iron concentration in mouse liver after acute and subacute cadmium intoxication. J Trace Elem Med Biol 22:66–72. https://doi.org/10.1016/j.jtemb.2007.09.024
Kingsley BS, Frazier JM (1979) Cadmium transport in isolated perfused rat liver: zinc-cadmium competition. Am J Physiol Physiol 236:C139–C143. https://doi.org/10.1152/ajpcell.1979.236.3.C139
Anstee QM, Goldin RD (2006) Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol 87:1–16. https://doi.org/10.1111/j.0959-9673.2006.00465.x
Malhi H, Guicciardi ME, Gores GJ (2010) Hepatocyte death: a clear and present danger. Physiol Rev 90:1165–1194. https://doi.org/10.1152/physrev.00061.2009
Wang J, Zhu H, Liu X, Liu Z (2014) Oxidative stress and Ca2+ signals involved on cadmium-induced apoptosis in rat hepatocyte. Biol Trace Elem Res 161:180–189. https://doi.org/10.1007/s12011-014-0105-6
Pham TND, Marion M, Denizeau F, Jumarie C (2006) Cadmium-induced apoptosis in rat hepatocytes does not necessarily involve caspase-dependent pathways. Toxicol Vitr 20:1331–1342. https://doi.org/10.1016/j.tiv.2006.05.005
Li Y, Lim SC (2007) Cadmium-induced apoptosis of hepatocytes is not associated with death receptor-related caspase-dependent pathways in the rat. Environ Toxicol Pharmacol 24:231–238. https://doi.org/10.1016/j.etap.2007.05.010
Torkzad MR, Norén A, Kullberg J (2012) Stereology: a novel technique for rapid assessment of liver volume. Insights Imaging 3:387–393. https://doi.org/10.1007/s13244-012-0166-z
Fernandez CDB, Porto EM, Arena AC, Kempinas WDG (2008) Effects of altered epididymal sperm transit time on sperm quality. Int J Androl 31:427–437. https://doi.org/10.1111/j.1365-2605.2007.00788.x
Habeebu SS (2000) Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol Sci 55:223–232. https://doi.org/10.1093/toxsci/55.1.223
Acknowledgments
The authors thank Bioclin Laboratories for kindly providing biochemical kits used in this work, and Enedina Sacramento for the English proofreading; this work was supported by Universidade Federal de Viçosa (UFV), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mouro, V.G.S., Ladeira, L.C.M., Lozi, A.A. et al. Different Routes of Administration Lead to Different Oxidative Damage and Tissue Disorganization Levels on the Subacute Cadmium Toxicity in the Liver. Biol Trace Elem Res 199, 4624–4634 (2021). https://doi.org/10.1007/s12011-020-02570-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02570-5