Abstract
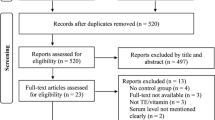
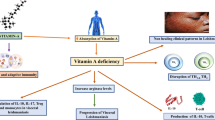
Leishmaniasis is a worldwide prevalent parasitic infection caused by different species of the genus Leishmania. Clinically, the disease divided into three main forms, including visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis (MCL). There is no vaccine for human leishmaniasis and their treatment is challenging. Trace elements (TEs) alteration, including the selenium (Se), zinc (Zn), copper (Cu), ron (Fe), and magnesium (Mg) have been detected in patients with CL and VL as well as canine leishmaniasis. Because TEs play a pivotal role in the immune system, and host immune responses have crucial roles in defense against leishmaniasis, this systematic review aimed to summarize data regarding TEs alteration in human and animal leishmaniasis as well as the role of these elements as an adjuvant for treatment of leishmaniasis. In a setting of systematic review, we found 29 eligible articles (any date until October 1, 2020) regarding TEs in human CL (N = 12), human VL (N = 4), canine leishmaniasis (N = 3), and treatment of leishmaniasis based on TEs (N = 11), which one study examined the TEs level both in CL and VL patients. Our analysis demonstrated a significantly decreased level of Fe, Zn, and Se among human CL and canine leishmaniasis, and Zn and Fe in patients with VL. In contrast, an increased level of Cu in CL patients and Cu and Mg in VL patients and canine leishmaniasis was observed. Treatment of CL based zinc supplementation revealed enhancement of wound healing and diminished scar formation in human and experimentally infected animals. The results of this systematic review indicate that the TEs have important roles in leishmaniasis, which could be assessed as a prognosis factor in this disease. It is suggested that TEs could be prescribed as an adjuvant for the treatment of CL and VL patients.

Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- VL:
-
visceral leishmaniasis
- CL:
-
cutaneous leishmaniasis
- MCL:
-
mucocutaneous leishmaniasis
- TEs:
-
trace elements
- Se:
-
selenium
- Zn:
-
zinc
- Cu:
-
copper
- Fe:
-
iron
- SOD:
-
superoxide dismutase
- GSH-Px:
-
glutathione peroxidase
- Gpx:
-
glutathione peroxidase enzyme
- Cp:
-
ceruloplasmin
- Htc:
-
hematocrit
- CAT:
-
catalase
- Th1:
-
T helper 1
- Th2:
-
T helper 2
- IFNγ:
-
interferon gamma
- TNF:
-
tumor necrosis factor
- TGF-β:
-
transforming growth factor beta
References
Gramiccia M, Gradoni L (2005) The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol 35(11-12):1169–1180
Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advances in leishmaniasis. Lancet. 366(9496):1561–1577
Bates PA (2007) Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol 37(10):1097–1106
Goto H, Lindoso JAL (2012) Cutaneous and mucocutaneous leishmaniasis. Infect Dis Clin 26(2):293–307
Ready PD (2014) Epidemiology of visceral leishmaniasis. Clin Epidemiol 6:147
Rodrigues V, Cordeiro-da-Silva A, Laforge M, Silvestre R, Estaquier J (2016) Regulation of immunity during visceral Leishmania infection. Parasit Vectors 9(1):118
Muller I, Pedrazzini T, Farrell JP, Louis J (1989) T-cell responses and immunity to experimental infection with Leishmania major. Annu Rev Immunol 7(1):561–578
Maspi N, Abdoli A, Ghaffarifar F (2016) Pro-and anti-inflammatory cytokines in cutaneous leishmaniasis: a review. Pathog Glob Health 110(6):247–260
Abdoli A, Maspi N, Ghaffarifar F (2017) Wound healing in cutaneous leishmaniasis: a double edged sword of IL-10 and TGF-β. Comp Immunol Microbiol Infect Dis 51:15–26. https://doi.org/10.1016/j.cimid.2017.02.001
O’Neal SE, Guimaraes LH, Machado PR, Alcântara L, Morgan DJ, Passos S et al (2007) Influence of helminth infections on the clinical course of and immune response to Leishmania braziliensis cutaneous leishmaniasis. J Infect Dis 195(1):142–148
Sarkar A, Saha P, Mandal G, Mukhopadhyay D, Roy S, Singh SK, Das S, Goswami RP, Saha B, Kumar D, Das P, Chatterjee M (2011) Monitoring of intracellular nitric oxide in leishmaniasis: its applicability in patients with visceral leishmaniasis. Cytometry A 79(1):35–45
Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR (2004) The immunopathology of experimental visceral leishmaniasis. Immunol Rev 201(1):239–253. https://doi.org/10.1111/j.0105-2896.2004.00188.x
Kima P, Soong L (2013) Interferon gamma in leishmaniasis. Front Immunol 4(156). https://doi.org/10.3389/fimmu.2013.00156
Blackwell JM, Fakiola M, Castellucci LC (2020) Human genetics of leishmania infections. Hum Genet 139(6):813–819. https://doi.org/10.1007/s00439-020-02130-w
Pinheiro RO, Rossi-Bergmann B (2007) Interferon-gamma is required for the late but not early control of Leishmania amazonensis infection in C57Bl/6 mice. Mem Inst Oswaldo Cruz 102(1):79–82
Kolde G, Luger T, Sorg C, Sunderkötter CS (1996) Successful treatment of cutaneous leishmaniasis using systemic interferon-gamma. Dermatology. 192(1):56–60. https://doi.org/10.1159/000246316
Badaro R, Falcoff E, Badaro FS, Carvalho EM, Pedral-Sampaio D, Barral A, Carvalho JS, Barral-Netto M, Brandely M, Silva L, Bina JC, Teixeira R, Falcoff R, Rocha H, Ho JL, Johnson WD Jr (1990) Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. N Engl J Med 322(1):16–21. https://doi.org/10.1056/nejm199001043220104
Sharma U, Singh S (2009) Immunobiology of leishmaniasis. Indian J Exp Biol 47(6):412–423
Wilhelm P, Ritter U, Labbow S, Donhauser N, Rollinghoff M, Bogdan C et al (2001) Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J Immunol 166(6):4012–4019
Garcia I, Miyazaki Y, Araki K, Araki M, Lucas R, Grau GE, Milon G, Belkaid Y, Montixi C, Lesslauer W, Vassalli P (1995) Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol 25(8):2401–2407. https://doi.org/10.1002/eji.1830250841
Scott P, Novais FO (2016) Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol 16(9):581–592. https://doi.org/10.1038/nri.2016.72
Underwood EJ (1977) Trace elements in human and animal nutrition. 1977 No.Ed. 4. Academic Press, Inc., London, UK
Kodama H (1996) Essential trace elements and immunity. Nihon Rinsho 54(1):46–51
Chandra RK, Dayton DH (1982) Trace element regulation of immunity and infection. Nutr Res 2(6):721–733
Amini M, Nahrevanian H, Khatami S, Farahmand M, Mirkhani F, Javadian S (2009) Biochemical association between essential trace elements and susceptibility to Leishmania major in BALB/c and C57BL/6 mice. Braz J Infect Dis 13(2):83–85
Faryadi M, Mohebali M (2003) Alterations of serum zinc, copper and iron concentrations in patients with acute and chronic cutaneous leishmaniasis. Iran J Public Health 32(4):53–58
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1
Farzin L, Moassesi ME, Sajadi F (2014) Alterations of serum antioxidant trace elements (Se, Zn and Cu) status in patients with cutaneous leishmaniasis. Asian Pac J Trop Dis 4:S445–S4S8
Pourfallah F, Javadian S, Zamani Z, Saghiri R, Sadeghi S, Zarea B, Faiaz Sh, Mirkhani F, Fatemi N (2009) Evaluation of serum levels of zinc, copper, iron, and zinc/copper ratio in cutaneous leishmaniasis. Iran J Arthropod Borne Dis 3(2):7–11
Farzin L, Moassesi ME (2014) A comparison of serum selenium, zinc and copper level in visceral and cutaneous leishmaniasis. J Res Med Sci 19(4):355–357
Kahvaz MS, Soltani S, Soltani S, Carvalheiro MC, Foroutan M (2020) Low serum levels of selenium, zinc, iron, and zinc/copper ratio in an endemic region of cutaneous leishmaniasis in southwest Iran. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02271-z
Koçyiğit A, Erel O, Gürel M, Avcı S, Aktepe N (1998) Serum selenium, zinc, copper and iron concentrations and some related antioxidant enzymes in patients with cutaneous leishmaniasis. Marmara Med J 11(2):77–82
Kocyigit A, Erel O, Seyrek A, Gurel M, Aktepe N, Avci S, Vural H (1998) Effects of antimonial therapy on serum zinc, copper and iron concentrations in patients with cutaneous Leishmaniasis in Turkey. J Egypt Soc Parasitol 28(1):133–142
Kocyigit A, Gur S, Erel O, Gurel MS (2002) Associations among plasma selenium, zinc, copper, and iron concentrations and immunoregulatory cytokine levels in patients with cutaneous leishmaniasis. Biol Trace Elem Res 90(1-3):47–55
Koçyiğit A, Erel Ö, Gürel MS, Seyrek A, Aktepe N, Gür S et al (1999) Decreasing selenium levels and glutathione peroxidase activity in patients with cutaneous leishmaniasis. Turk J Med Sci 29(3):291–296
Van Weyenbergh J, Santana G, D'Oliveira A, Santos AF, Costa CH, Carvalho EM et al (2004) Zinc/copper imbalance reflects immune dysfunction in human leishmaniasis: an ex vivo and in vitro study. BMC Infect Dis 4(1):50
Al-Hassani MKK, Al-Mayali HMH (2020) Evaluation of some biochemical levels in patients with Cutaneous leishmaniasis serum and their relationship with antioxidant enzymes. EurAsian J Biosci 14(1):1999–2006
Najafzade M, Mosapour A, Nahrevanian H, Zamani Z, Javadian S, Mirkhani F (2015) Effect of trinitroglycerin therapy on serum zinc and copper levels and liver enzyme activities in BALB/c mice infected with Leishmania major MRHO/IR/75/ER. Iran J Basic Med Sci 18(3):77–283
Mishra J, Carpenter S, Singh S (2010) Low serum zinc levels in an endemic area of visceral leishmaniasis in Bihar, India. Indian J Med Res 131(6):793–798
Lal CS, Kumar S, Ranjan A, Rabidas VN, Verma N, Pandey K, Verma RB, Das S, Singh D, Das P (2013) Comparative analysis of serum zinc, copper, magnesium, calcium and iron level in acute and chronic patients of visceral leishmaniasis. J Trace Elem Med Biol 27(2):98–102
Souza CC, de O Barreto T, da Silva SM, Pinto AW, Figueiredo MM, Ferreira Rocha OG et al (2014) A potential link among antioxidant enzymes, histopathology and trace elements in canine visceral leishmaniasis. Int J Exp Pathol 95(4):260–270
Heidarpour M, Soltani S, Mohri M, Khoshnegah J (2012) Canine visceral leishmaniasis: relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol Res 111(4):1491–1496
Pasa S, Kargin F, Bildik A, Seyrek K, Ozbel Y, Ozensoy S (2003) Serum and hair levels of zinc and other elements in dogs with visceral leishmaniasis. Biol Trace Elem Res 94(2):141–147. https://doi.org/10.1385/BTER:94:2:141
Sharquie K, Najim R, Farjou I, Al-Timimi D (2001) Oral zinc sulphate in the treatment of acute cutaneous leishmaniasis. Clin Exp Dermatol 26(1):21–26
Sharquie K, Najim R, Farjou I (1997) A comparative controlled trial of intralesionally-administered zinc sulphate, hypertonic sodium chloride and pentavalent antimony compound against acute cutaneous leishmaniasis. Clin Exp Dermatol 22(4):169–173
Sharquie KE, Noaimi AA, Sharara ZA, Saleh BA, Al-Salam WS (2017) Topical therapy of acute cutaneous leishmaniasis using zinc sulphate solution 25% versus podophyllin solution 25%. J Chem Dermatol Sci Appl 7(03):258–274
Sharquie KE, Noaimi AA, Al-Salam WS (2016) Treatment of acute cutaneous Leishmaniasis by oral zinc sulfate and oral ketocanazole singly and in combination. J Chem Dermatol Sci Appl 6(03):105
Carbone DCB, Zanoni LZG, Cônsolo FZ, Sanches SC, Quadros dos Reis V, de Toledo Candido Muller K et al (2018) Potential role of zinc in the visceromegaly regression and recovery of hematological parameters during treatment of visceral leishmaniasis in children from an endemic area. Rev Inst Med Trop Sao Paulo 60:1–7
Farajzadeh S, Ahmadi R, Mohammadi S, Pardakhty A, Khalili M, Aflatoonian M (2018) Evaluation of the efficacy of intralesional Glucantime plus niosomal zinc sulphate in comparison with intralesional Glucantime plus cryotherapy in the treatment of acute cutaneous leishmaniasis, a randomized clinical trial. J Parasit Dis 42(4):616–620. https://doi.org/10.1007/s12639-018-1044-5
Firooz A, Khatami A, Khamesipour A, Nassiri-Kashani M, Behnia F, Nilforoushzadeh M et al (2005) Intralesional injection of 2% zinc sulfate solution in the treatment of acute old world cutaneous leishmaniasis: a randomized, double-blind, controlled clinical trial. J Drugs Dermatol 4(1):73–79
Maleki M, Karimi G, Tafaghodi M, Raftari S, Nahidi Y (2012) Comparison of intralesional two percent zinc sulfate and glucantime injection in treatment of acute cutaneous leishmaniasis. Indian J Dermatol 57(2):118–122
Sorkhroodi FZ, Naeini AA, Ramazani AZ, Ghazvini MA, Mohebali M, Keshavarz S (2010) Therapeutic effect of sodium selenite and zinc sulphate as supplementary with meglumine antimoniate (glucantime®) against cutaneous leishmaniasis in BALB/c mice. Iran J Parasitol 5(3):11–19
Afshari M, Riazi-Rad F, Khaze V, Bahrami F, Ajdary S, Alimohammadian MH (2016) Oral treatment with zinc sulfate increases the expression of Th1 cytokines mRNA in BALB/c mice infected with Leishmania major. Cytokine. 81:71–76. https://doi.org/10.1016/j.cyto.2016.02.002
Najim RA, Sharquie KE, Farjou IB (1998) Zinc sulphate in the treatment of cutaneous leishmaniasis: an in vitro and animal study. Mem Inst Oswaldo Cruz 93(6):831–837
Antinori S, Schifanella L, Corbellino M (2012) Leishmaniasis: new insights from an old and neglected disease. Eur J Clin Microbiol Infect Dis 31(2):109–118. https://doi.org/10.1007/s10096-011-1276-0
Nweze JA, Nweze EI, Onoja US (2020) Nutrition, malnutrition, and leishmaniasis. Nutrition. 73:110712. https://doi.org/10.1016/j.nut.2019.110712
Failla ML (2003) Trace elements and host defense: recent advances and continuing challenges. J Nutr 133(5):1443S–1447S. https://doi.org/10.1093/jn/133.5.1443S
Dryden M (2018) Reactive oxygen species: a novel antimicrobial. Int J Antimicrob Agents 51(3):299–303. https://doi.org/10.1016/j.ijantimicag.2017.08.029
(1981) Severe zinc deficiency in humans: association with a reversible T-lymphocyte dysfunction. Ann Intern Med 95(2):154–157. https://doi.org/10.7326/0003-4819-95-2-154
Beck F, Prasad A, Kaplan J, Fitzgerald J, Brewer G (1997) Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol Endocrinol Metab 272(6):E1002–E10E7
Foster M, Samman S (2012) Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients 4(7):676–694. https://doi.org/10.3390/nu4070676
Bao B, Prasad AS, Beck FWJ, Godmere M (2003) Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab 285(5):E1095–EE102. https://doi.org/10.1152/ajpendo.00545.2002
Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD et al (2007) Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 85(3):837–844. https://doi.org/10.1093/ajcn/85.3.837
Lin P-H, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J (2018) Zinc in wound healing modulation. Nutrients 10(1):16. https://doi.org/10.3390/nu10010016
Kogan S, Sood A, Garnick MS (2017) Zinc and wound healing: a review of zinc physiology and clinical applications. Wounds 29(4):102–106
Cassat James E, Skaar EP (2013) Iron in infection and immunity. Cell Host Microbe 13(5):509–519. https://doi.org/10.1016/j.chom.2013.04.010
Nairz M, Weiss G (2020) Iron in infection and immunity. Mol Asp Med 75:100864. https://doi.org/10.1016/j.mam.2020.100864
Ganz T (2018) Iron and infection. Int J Hematol 107(1):7–15. https://doi.org/10.1007/s12185-017-2366-2
Laranjeira-Silva MF, Hamza I, Pérez-Victoria JM (2020) Iron and heme metabolism at the leishmania–host interface. Trends Parasitol 36(3):279–289. https://doi.org/10.1016/j.pt.2019.12.010
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Soflaei S, Dalimi A, Abdoli A, Kamali M, Nasiri V, Shakibaie M, Tat M (2014) Anti-leishmanial activities of selenium nanoparticles and selenium dioxide on Leishmania infantum. Comp Clin Pathol 23(1):15–20. https://doi.org/10.1007/s00580-012-1561-z
Beheshti N, Soflaei S, Shakibaie M, Yazdi MH, Ghaffarifar F, Dalimi A, Shahverdi AR (2013) Efficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studies. J Trace Elem Med Biol 27(3):203–207. https://doi.org/10.1016/j.jtemb.2012.11.002
Mostafavi M, Farajzadeh S, Sharifi I, Khazaeli P, Sharifi H (2019) Leishmanicidal effects of amphotericin B in combination with selenium loaded on niosome against Leishmania tropica. J Parasit Dis 43(2):176–185. https://doi.org/10.1007/s12639-018-1071-2
Mostafavi M, Khazaeli P, Sharifi I, Farajzadeh S, Sharifi H, Keyhani A, Parizi MH, Kakooei S (2019) A novel niosomal combination of selenium coupled with glucantime against Leishmania tropica. Korean J Parasitol 57(1):1–8
Percival SS (1998) Copper and immunity. Am J Clin Nutr 67(5):1064S–1068S. https://doi.org/10.1093/ajcn/67.5.1064S
Kubenam KS (1994) The role of magnesium in immunity. J Nutr Immunol 2(3):107–126. https://doi.org/10.1300/J053v02n03_07
Whitehouse MW, Walker WR (1978) Copper and inflammation. Agents Actions 8(1):85–90. https://doi.org/10.1007/BF01972407
Lv J, Xiao Q, Chen Y, Fan X, Liu X, Liu F, Luo G, Zhang B, Wang S (2017) Effects of magnesium isoglycyrrhizinate on AST, ALT, and serum levels of Th1 cytokines in patients with allo-HSCT. Int Immunopharmacol 46:56–61. https://doi.org/10.1016/j.intimp.2017.02.022
Han F, Xu L, Huang Y, Chen T, Zhou T, Yang L (2018) Magnesium sulphate can alleviate oxidative stress and reduce inflammatory cytokines in rat placenta of intrahepatic cholestasis of pregnancy model. Arch Gynecol Obstet 298(3):631–638. https://doi.org/10.1007/s00404-018-4850-1
Nielsen FH (2018) Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res 11:25–34. https://doi.org/10.2147/JIR.S136742
Funding
Amir Abdoli is supported by National Institute for Medical Research Development (NIMAD) grant number: 978507. This study is financially purported by Jahrom University of Medical Sciences, Jahrom, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taghipour, A., Abdoli, A., Ramezani, A. et al. Leishmaniasis and Trace Element Alterations: a Systematic Review. Biol Trace Elem Res 199, 3918–3938 (2021). https://doi.org/10.1007/s12011-020-02505-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02505-0