Abstract
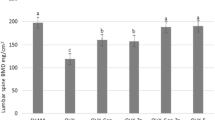
We have reported that genistein (Gen) and silicon (Si) have synergistic effects on ovariectomy-induced bone loss in rat; however, the potential mechanisms behind this effect were not fully clarified yet. This study was performed to evaluate the bone protective mechanisms of concomitant intake of genistein and silicon in ovariectomized rat by OPG/RANKL axis. Three-month-old Sprague-Dawley female rats were subjected to ovariectomy (OVX) or sham surgery; after surgery, the OVX rats were randomly divided into five groups: OVX-Gen, OVX-Si, OVX-Gen-Si, OVX-E, and OVX. Genistein, silicon, and 17β-estradiol supplementation were started after ovariectomy and continued for 10 weeks. The results showed that genistein and silicon treatment increased the bone mineral density (BMD) of ovariectomized rats. In addition, the BMD of the tibia and femur were highest in the OVX-Gen-Si group compared with OVX-Gen and OVX-Si group (p < 0.05). After 10 weeks treatment with genistein and silicon, the bone structure of ovariectomized rats was recovered, there was no difference of bone histomorphometric parameters between OVX-Gen-Si, OVX-E, and SHAM group (p > 0.05), and there was no difference in the concentration of serum ALP, Ca, P, OPG, and RANKL between OVX-Gen-Si, SHAM, and OVX-E groups (p > 0.05). RT-PCR showed that genistein and silicon treatment could effectively increase the OPG mRNA expression and decreased the RANKL mRNA expression compared to that of the OVX group (p < 0.05), the OPG/RANKL mRNA ratios were significantly decreased in the OVX group (p < 0.05), and it was nearly to normal in the OVX-Gen-Si group. Immunohistochemical staining results showed that genistein and silicon supplementation could effectively increase the protein expression of OPG and decrease the protein expression of RANKL in bone tissues; there were no significant differences in OPG and RANKL positive expression areas between OVX-Gen-Si, SHAM, and OVX-E group (p > 0.05). The results above indicate that genistein and silicon supplementation can effectively reduce RANKL, increase OPG levels, and OPG/RANKL ratios in the serum and bone tissue of ovariectomized rats; this is the main mechanism by which genistein and silicon play a bone protective role in ovariectomized rats.







Similar content being viewed by others
References
Bijelic R, Milicevic S, Balaban J (2017) Risk factors for osteoporosis in postmenopausal women. Mediev Archaeol 71:25–28
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287
Sun FF, Shi HR (2014) Progress of hormone replacement therapy and breast cancer. Int J Gynecol Obstet 41:146–149
Marsden J (2002) The menopause, hormone replacement therapy and breast cancer. J Steroid Biochem Mol Biol 83(1):123–132
Miraj S (2016) Scientific basis for the therapeutic use of soybean: a review study. Pharm Lett 8(19):421–425
Levis S, Strickman S, Ganjei P et al (2011) Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med 171:1363–1369
Messina M (2014) Soy foods, isoflavones, and the health of postmenopausal women. Am J Clin Nutr 100(1):423S
Zhang X, Shu XO, Li H et al (2005) Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med 165:1890–1895
Gail AG, Gordon FG, Huang MH et al (2002) Dietary soy isoflavones and bone mineral density: results from the study of women’s health across the nation. Am J Epidemiol 15(8):746–754
Qi SS (2018) Synergistic effects of genistein and zinc on bone metabolism and the femoral metaphyseal histomorphology in the ovariectomized rats. Biol Trace Elem Res 183(2):288–295
Jugdaohsingh R (2007) Silicon and bone health. J Nutr Health Aging 11:99–110
Bae YJ, Kim JY, Choi MK, Chung YS, Kim MH (2008) Short-term administration of water-soluble silicon improves mineral density of the femur and tibia in ovariectomized rats. Biol Trace Elem Res 124:157–163
Kim MH, Bae YJ, Choi MK, Chung YS (2009) Silicon supplementation improves the bone mineral density of calcium-deficient ovariectomized rats by reducing bone resorption. Biol Trace Elem Res 128:239–247
Jugdaohsingh R, Tucker KL, Qiao N (2010) Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the framingham offspring cohort. J Bone Miner Res 19(2):297–307
Khosla S, Melton LJ, Riggs BL (2011) The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res 26:441–451
Qi SS, Zheng HX (2017) Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res 177(2):281–285
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 15(2):139–146
Anna E, Silvia RG, Pilar P et al (2010) The effect of the alendronate on OPG/RANKL system in differentiated primary human osteoblasts. Endocrine 37(2):322–328
Liu JM, Zhao HY, Ning G et al (2005) Relationships between the changes of serum levels of OPG and RANKL with age, menopause, bone biochemical markers and bone mineral density in Chinese women aged 20–75. Calcif Tissue Int 76(1):1–6
Hao Y, Gao R, Lu B et al (2018) Ghrelin protects against depleted uranium-induced bone damage by increasing osteoprotegerin/RANKL ratio. Toxicol Appl Pharmacol 343(15):62–70
Bin J, Li X, Qi Z et al (2014) A hypomagnetic field aggravates bone loss induced by hindlimb unloading in rat Femurs. PLoS One 9(8):e105604
Seeman E (2004) Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep 2(3):90–96
Zhou L, Liu Q, Yang M et al (2016) Dihydroartemisinin, an anti-malaria drug, suppresses estrogen deficiency-induced osteoporosis, osteoclast formation, and RANKL-induced signaling pathways. J Bone Miner Res 31(5):964–970
Persson I, Bergkvist L, Lindgren C et al (1997) Hormone replacement therapy and major risk factors for reproductive cancers, osteoporosis, and cardiovascular diseases: evidence of confounding by exposure characteristics. J Clin Epidemiol 50(5):611–618
Anazi AF, Qureshi VF, Javaid K (2011) Preventive effects of phytoestrogens against postmenopausal osteoporosis as compared to the available therapeutic choices: an overview. J Nat Sci Biol Med 2(2):154–163
Listed N (2007) Effects of the phytoestrogen genistein on bone health in postmenopausal women. Ann Intern Med 146(12):134–137
Hott M, Pollak C, Modrowski D et al (1993) Short-term effects of organic silicon on trabecular bone in mature ovariectomized rats. Calcif Tissue Int 53:174–179
Hofbauer LC, Khosla S, Dunstan CR et al (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15(1):2–12
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473(2):139–146
Crockett JC, Mellis DJ, Scott DI et al (2011) New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis. Osteoporos Int 22(1):1–20
Wang J, He M, Wang G et al (2017) Organic gallium treatment improves osteoporotic fracture healing through affecting the OPG/RANKL ratio and expression of serum inflammatory cytokines in ovariectomized rats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-017-1123-y
Lacey DL, Boyle WJ, Simonet WS et al (2012) Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov 11(5):401–419
Gonzalo SD, Christian H, Petra K et al (2015) Bone morphogenetic protein signaling in bone homeostasis. Bone 80:43–59
Karst M, Gorny G, Galvin RJ et al (2004) Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J Cell Physiol 200(1):99–106
Komori T (2011) Signaling networks in RUNX2-dependent bone development. J Cell Biochem 112(3):750–758
Gade TP, Motley MW, Beattie BJ et al (2011) Imaging of alkaline phosphatase activity in bone tissue. PLoS ONE 6(7):e22608
Watts NB, Jenkins DK, Visor JM et al (2001) Comparison of bone and total alkaline phosphatase and bone mineral density in postmenopausal osteoporotic women treated with alendronate. Osteoporos Int 12:279–288
Mukaiyama K, Kamimura M, Uchiyama S et al (2015) Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res 27:413–418
Thompson WR, Rubin CT, Rubin J (2012) Mechanical regulation of signaling pathways in bone. Gene 503(2):179–193
Day TF, Yang Y (2008) Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am 90(1):19–24
Funding
This work was supported by the High-end Foreign Experts Recruitment Programme of State Administration of Foreign Experts Affairs (GDT20176100048), the Key Project of Agricultural Science and Technology of Shaanxi Province (2017NY-082), Shaanxi Science and Technology Coordinating Innovation Engineering Program (2015KTTSSF01–03, 2015HBGC-18), Shaanxi Province Key Research and Development Plan (2017SF-074), Research Project of Shaanxi Provincial Education Department (17JK0135), Postodoctoral Project of Shaanxi University of Technology (SLGBH16-03), and Qinling-Bashan Mountains Bioresources Comprehensive Development, Collaborative Innovation Center Research Funds (QBXT-17-9).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, C., Zheng, H. & Qi, S. Genistein and Silicon Synergistically Protects Against Ovariectomy-Induced Bone Loss Through Upregulating OPG/RANKL Ratio. Biol Trace Elem Res 188, 441–450 (2019). https://doi.org/10.1007/s12011-018-1433-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1433-8