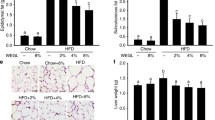
Abstract
Diabetes mellitus (DM), a metabolic and endocrine condition, poses a serious threat to human health and longevity. The emerging role of gut microbiome associated with bioactive compounds has recently created a new hope for DM treatment. UHPLC-HRMS methods were used to identify these compounds in a poly herbal ethanolic extract (PHE). The effects of PHE on body weight (BW), fasting blood glucose (FBG) level, gut microbiota, fecal short-chain fatty acids (SCFAs) production, and the correlation between DM-related indices and gut microbes, in rats were investigated. Chebulic acid (0.368%), gallic acid (0.469%), andrographolide (1.304%), berberine (6.442%), and numerous polysaccharides were the most representative constituents in PHE. A more significant BW gain and a reduction in FBG level towards normal of PHE 600 mg/kg treated rats group were resulted at the end of 28th days of the study. Moreover, the composition of the gut microbiota corroborated the study’s hypothesis, as evidenced by an increased ratio of Bacteroidetes to Firmicutes and some beneficial microbial species, including Prevotella copri and Lactobacillus hamster. The relative abundance of Bifidobacterium pseudolongum, Ruminococcus bromii, and Blautia producta was found to decline in PHE treatment groups as compared to diabetic group. The abundance of beneficial bacteria in PHE 600 mg/kg treatment group was concurrently associated with increased SCFAs concentrations of acetate and propionate (7.26 nmol/g and 4.13 nmol/g). The findings of this study suggest a promising approach to prevent DM by demonstrating that these naturally occurring compounds decreased FBG levels by increasing SCFAs content and SCFAs producing gut microbiota.
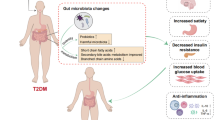
Graphical Abstract
Flow chart summarizing research on the dual therapeutic approach to diabetes mellitus via bioactive chemicals found in a poly herbal extract and the management of gut microbiota in relation to DM. (SCFAs, short chain fatty acids; SMB53, a genus of bacterial microbiota of small intestine; LPS, lipopolysaccharide)










Similar content being viewed by others
Data Availability
Not applicable.
References
Bunsroem, K., Prinyawiwatkul, W., & Thaiudom, S. (2022). The influence of whey protein heating parameters on their susceptibility to digestive enzymes and the antidiabetic activity of hydrolysates. Foods, 11(6), 829. https://doi.org/10.3390/foods11060829
Inayati, A., Lee, B. O., Wang, R. H., Chen, S. Y., Hsu, H. C., Lu, C. H., & Lee, Y. J. (2022). Determinants of fear of falling in older adults with diabetes. Geriatric Nursing, 46, 7–12. https://doi.org/10.1016/j.gerinurse.2022.04.017
Singh, A. K., Kumar, P., Rajput, V. D., Mishra, S. K., Tiwari, K. N., Singh, A. K., Minkina, T., & Pandey, A. K. (2023). Phytochemicals, antioxidant, anti-inflammatory studies, and identification of bioactive compounds using GC-MS of ethanolic novel polyherbal extract. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-023-04363-7
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., Stein, C., Basit, A., Chan, J. C. N., Mbanya, J. C., Pavkov, M. E., Ramachandaran, A., Wild, S. H., James, S., Herman, W. H., Zhang, P., Bommer, C., Kuo, S., Boyko, E. J., & Magliano, D. J. (2022). IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice, 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119
Lorenzati, B., Zucco, C., Miglietta, S., Lamberti, F., & Bruno, G. (2010). Oral hypoglycemic drugs: Pathophysiological basis of their mechanism of actionoral hypoglycemic drugs: Pathophysiological basis of their mechanism of action. Pharmaceuticals, 3, 3005–3020. https://doi.org/10.3390/ph3093005
Calcutt, N. A., Cooper, M. E., Kern, T. S., & Schmidt, A. M. (2009). Therapies for hyperglycaemia-induced diabetic complications: From animal models to clinical trials. Nature Reviews. Drug Discovery, 8(5), 417–429. https://doi.org/10.1038/nrd2476
Jaiswal, Y. S., & Williams, L. L. (2017). A glimpse of Ayurveda-The forgotten history and principles of Indian traditional medicine. Journal of Traditional and Complementary Medicine, 7(1), 50–53.
Rathor, L., Pant, A., Awasthi, H., Mani, D., & Pandey, R. (2017). An antidiabetic polyherbal phytomedicine confers stress resistance and extends lifespan in Caenorhabditis elegans. Biogerontology, 18, 131–147.
Srivastava, S., Lal, V. K., & Pant, K. K. (2012). Polyherbal formulations based on Indian medicinal plants as antidiabetic phytotherapeutics. Phytopharmacology, 2(1), 1–15.
Girish, S., Kuber, S., & Nataraj, H. R. (2015). Review on Kataka (Strychnous potatorum Linn). International Journal of Research in Ayurveda and Pharmacy, 6(1), 86–89.
Duraiswamy, A., Shanmugasundaram, D., Sasikumar, C. S., Cherian, S. M., & Cherian, K. M. (2015). Development of an antidiabetic formulation (ADJ6) and its inhibitory activity against α-amylase and α-glucosidase. Journal of Traditional and Complementry Medicine, 6(3), 204–208. https://doi.org/10.1016/j.jtcme.2014.12.006
Kumudhaveni, B., & Radha, R. (2017). Anti-diabetic potential of a traditional Polyherbal formulation-A review. Research Journal of Pharmacy and Technology, 10(6), 1865–1869.
Wediasari, F., Nugroho, G. A., Fadhilah, Z., Elya, B., Setiawan, H., & Mozef, T. (2020). Hypoglycemic effect of a combined Andrographis paniculata and Caesalpinia sappan extract in streptozocin-induced diabetic rats. Advances in Pharmacological and Pharmaceutical Sciences, 2020, 8856129. https://doi.org/10.1155/2020/8856129
Piccolella, S., Crescente, G., Candela, L., & Pacifico, S. (2019). Nutraceutical polyphenols: New analytical challenges and opportunities. Journal of Pharmaceutical and Biomedical Analysis, 175, 112774. https://doi.org/10.1016/j.jpba.2019.07.022
Turi, C. E., Finley, J., Shipley, P. R., Murch, S. J., & Brown, P. N. (2015). Metabolomics for phytochemical discovery: Development of statistical approaches using a cranberry model system. Journal of Natural Products, 78(4), 953–966. https://doi.org/10.1021/np500667z
Brahmi-Chendouh, N., Piccolella, S., Crescente, G., Pacifico, F., Boulekbache, L., Hamri-Zeghichi, S., Akkal, S., Madani, K., & Pacifico, S. (2019). A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. Journal of Food and Drug Analysis, 27(3), 692–702. https://doi.org/10.1016/j.jfda.2018.11.006
Makki, K., Deehan, E. C., Walter, J., & Bäckhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell Host and Microbe, 23(6), 705–715. https://doi.org/10.1016/j.chom.2018.05.012
Sharma, S., & Tripathi, P. (2019). Gut microbiome and type 2 diabetes: Where we are and where to go? The Journal of Nutritional Biochemistry, 63, 101–108. https://doi.org/10.1016/j.jnutbio.2018.10.003
Xu, W. T., Nie, Y. Z., Yang, Z., & Lu, N. H. (2016). The crosstalk between gut microbiota and obesity and related metabolic disorders. Future Microbiology, 11, 825–836. https://doi.org/10.2217/fmb-2015-0024
Singer-Englar, T., Barlow, G., & Mathur, R. (2019). Obesity, diabetes, and the gut microbiome: An updated review. Expert Review of Gastroenterology and Hepatology, 13(1), 3–15. https://doi.org/10.1080/17474124.2019.1543023
Puddu, A., Sanguineti, R., Montecucco, F., & Viviani, G. L. (2014). Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators of Inflammation, 2014, 162021. https://doi.org/10.1155/2014/162021
Ley, R. E., Bäckhed, F., Turnbaugh, P., Lozupone, C. A., Knight, R. D., & Gordon, J. I. (2005). Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America, 102(31), 11070–11075. https://doi.org/10.1073/pnas.0504978102
Kreznar, J. H., Keller, M. P., Traeger, L. L., Rabaglia, M. E., Schueler, K. L., Stapleton, D. S., Zhao, W., Vivas, E. I., Yandell, B. S., Broman, A. T., Hagenbuch, B., Attie, A. D., & Rey, F. E. (2017). Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell reports, 18(7), 1739–1750. https://doi.org/10.1016/j.celrep.2017.01.062
Drissi, F., Buffet, S., Raoult, D., & Merhej, V. (2015). Common occurrence of antibacterial agents in human intestinal microbiota. Frontiers in Microbiology, 6, 441. https://doi.org/10.3389/fmicb.2015.00441
Federici, M. (2019). Gut microbiome and microbial metabolites: A new system affecting metabolic disorders. Journal of Endocrinological Investigation, 42(9), 1011–1018. https://doi.org/10.1007/s40618-019-01022-9
Salgaço, M. K., Oliveira, L. G. S., Costa, G. N., Bianchi, F., & Sivieri, K. (2019). Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Applied Microbiology and Biotechnology, 103(23–24), 9229–9238. https://doi.org/10.1007/s00253-019-10156-y
Fiorucci, S., & Distrutti, E. (2015). Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends in Molecular Medicine, 21(11), 702–714. https://doi.org/10.1016/j.molmed.2015.09.001
Cani, P. D., Plovier, H., Van Hul, M., Geurts, L., Delzenne, N. M., Druart, C., & Everard, A. (2016). Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nature Reviews. Endocrinology, 12(3), 133–143. https://doi.org/10.1038/nrendo.2015.211
Sohail, M. U., Althani, A., Anwar, H., Rizzi, R., & Marei, H. E. (2017). Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. Journal of Diabetes Research, 2017, 9631435. https://doi.org/10.1155/2017/9631435
Larsen, N., Vogensen, F. K., van den Berg, F. W., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., Al-Soud, W. A., Sørensen, S. J., Hansen, L. H., & Jakobsen, M. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE, 5(2), e9085. https://doi.org/10.1371/journal.pone.0009085
Wei, X., Tao, J., Xiao, S., Jiang, S., Shang, E., Zhu, Z., Qian, D., & Duan, J. (2018). Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Scientific Reports, 8(1), 3685. https://doi.org/10.1038/s41598-018-22094-2
Forslund, K., Hildebrand, F., Nielsen, T., Falony, G., Le Chatelier, E., Sunagawa, S., Prifti, E., Vieira-Silva, S., Gudmundsdottir, V., Pedersen, H. K., Arumugam, M., Kristiansen, K., Voigt, A. Y., Vestergaard, H., Hercog, R., Costea, P. I., Kultima, J. R., Li, J., Jørgensen, T., Levenez, F., … Pedersen, O. (2015). Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature, 528(7581), 262–266. https://doi.org/10.1038/nature15766
Harbison, J. E., Roth-Schulze, A. J., Giles, L. C., Tran, C. D., Ngui, K. M., Penno, M. A., Thomson, R. L., Wentworth, J. M., Colman, P. G., Craig, M. E., Morahan, G., Papenfuss, A. T., Barry, S. C., Harrison, L. C., & Couper, J. J. (2019). Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatric diabetes, 20(5), 574–583. https://doi.org/10.1111/pedi.12865
van Passel, M. W., Kant, R., Zoetendal, E. G., Plugge, C. M., Derrien, M., Malfatti, S. A., Chain, P. S., Woyke, T., Palva, A., de Vos, W. M., & Smidt, H. (2011). The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS ONE, 6(3), e16876. https://doi.org/10.1371/journal.pone.0016876
Yan, Z., Wu, H., Zhou, H., Chen, S., He, Y., Zhang, W., Chen, T., Yao, H., & Su, W. (2020). Integrated metabolomics and gut microbiome to the effects and mechanisms of naoxintong capsule on type 2 diabetes in rats. Scientific reports, 10(1), 10829. https://doi.org/10.1038/s41598-020-67362-2
Salamone, D., Rivellese, A. A., & Vetrani, C. (2021). The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetologica, 58(9), 1131–1138.
Abu-Reidah, I. M., Critch, A. L., Manful, C. F., Rajakaruna, A., Vidal, N. P., Pham, T. H., Cheema, M., & Thomas, R. (2021). Effects of pH and temperature on water under pressurized conditions in the extraction of nutraceuticals from Chaga (Inonotus obliquus) Mushroom. Antioxidants, 10(8), 1322. https://doi.org/10.3390/antiox10081322
Hilary, S., Mohamed, O., Platat, C., Qureshi, M. A., Kizhakkayil, J., Al-Meqbaali, F., & Howarth, F. C. (2023). Supplemental ferulic acid does not affect metabolic markers and improves some oxidative damage parameters in diabetic rats. Heliyon, 9(6), e17313. https://doi.org/10.1016/j.heliyon.2023.e17313
Ojo, O. A., Amanze, J. C., Oni, A. I., Grant, S., Iyobhebhe, M., Elebiyo, T. C., Rotimi, D., Asogwa, N. T., Oyinloye, B. E., Ajiboye, B. O., & Ojo, A. B. (2022). Antidiabetic activity of avocado seeds (Persea americana Mill.) in diabetic rats via activation of PI3K/AKT signaling pathway. Scientific Reports, 12(1), 2919. https://doi.org/10.1038/s41598-022-07015-8
Klindworth, A., Pruesse, E., Schweer, T., Peplies, J., Quast, C., Horn, M., & Glöckner, F. O. (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Research, 41(1), e1. https://doi.org/10.1093/nar/gks808
DeSantis, T. Z., Hugenholtz, P., Larsen, N., Rojas, M., Brodie, E. L., Keller, K., Huber, T., Dalevi, D., Hu, P., & Andersen, G. L. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology, 72(7), 5069–5072. https://doi.org/10.1128/AEM.03006-05
Sasaki, D., Sasaki, K., Ikuta, N., Yasuda, T., Fukuda, I., Kondo, A., & Osawa, R. (2018). Low amounts of dietary fibre increase in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Scientific Reports, 8(1), 435. https://doi.org/10.1038/s41598-017-18877-8
Seçkin, H. (2022). Microflora in rats metagenomic analysis of intestinal microflora in rats treated with ellagic acid and sinapic acid using 16 S rDNA gene region. Indian Journal of Pharmaceutical Education and Research, 56(3).
Kim, D. H., Sim, Y., Hwang, J. H., Kwun, I. S., Lim, J. H., Kim, J., Kim, J. I., Baek, M. C., Akbar, M., Seo, W., Kim, D. K., Song, B. J., & Cho, Y. E. (2021). Ellagic acid prevents binge alcohol-induced leaky gut and liver injury through inhibiting gut dysbiosis and oxidative stress. Antioxidants (Basel, Switzerland), 10(9), 1386. https://doi.org/10.3390/antiox10091386
Chao, J., Huo, T. I., Cheng, H. Y., Tsai, J. C., Liao, J. W., Lee, M. S., Qin, X. M., Hsieh, M. T., Pao, L. H., & Peng, W. H. (2014). Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE, 9(2), e96969. https://doi.org/10.1371/journal.pone.0096969
Brahmachari, G. (Ed.). (2017). Discovery and development of neuroprotective agents from natural products. Elsevier.
Han, J., Lin, H., & Huang, W. (2011). Modulating gut microbiota as an anti-diabetic mechanism of berberine. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 17(7), RA164–RA167.
Cheng, H., Liu, J., Tan, Y., Feng, W., & Peng, C. (2022). Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. Journal of pharmaceutical analysis, 12(4), 541–555. https://doi.org/10.1016/j.jpha.2021.10.003
Li, C., Ai, G., Wang, Y., Lu, Q., Luo, C., Tan, L., Lin, G., Liu, Y., Li, Y., Zeng, H., Chen, J., Lin, Z., Xian, Y., Huang, X., Xie, J., & Su, Z. (2020). Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacological Research, 152, 104603. https://doi.org/10.1016/j.phrs.2019.104603
Bian, Y., Lei, J., Zhong, J., Wang, B., Wan, Y., Li, J., Liao, C., He, Y., Liu, Z., Ito, K., & Zhang, B. (2022). Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. The Journal of Nutritional Biochemistry, 99, 108840. https://doi.org/10.1016/j.jnutbio.2021.108840
Etxeberria, U., Arias, N., Boqué, N., Macarulla, M. T., Portillo, M. P., Martínez, J. A., & Milagro, F. I. (2015). Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. The Journal of Nutritional Biochemistry, 26(6), 651–660. https://doi.org/10.1016/j.jnutbio.2015.01.002
Qiao, Y., Zhang, Z., Zhai, Y., Yan, X., Zhou, W., Liu, H., Guan, L., & Peng, L. (2022). Apigenin alleviates obesity-associated metabolic syndrome by regulating the composition of the gut microbiome. Frontiers in Microbiology, 12, 805827. https://doi.org/10.3389/fmicb.2021.805827
Ge, X., He, X., Liu, J., Zeng, F., Chen, L., Xu, W., Shao, R., Huang, Y., Farag, M. A., Capanoglu, E., El-Seedi, H. R., Zhao, C., & Liu, B. (2023). Amelioration of type 2 diabetes by the novel 6, 8-guanidyl luteolin quinone-chromium coordination via biochemical mechanisms and gut microbiota interaction. Journal of Advanced Research, 46, 173–188. https://doi.org/10.1016/j.jare.2022.06.003
Ge, X., Wang, C., Chen, H., Liu, T., Chen, L., Huang, Y., Zeng, F., & Liu, B. (2020). Luteolin cooperated with metformin hydrochloride alleviates lipid metabolism disorders and optimizes intestinal flora compositions of high-fat diet mice. Food and Function, 11(11), 10033–10046. https://doi.org/10.1039/d0fo01840f
Cai, C., Cheng, W., Shi, T., Liao, Y., Zhou, M., & Liao, Z. (2023). Rutin alleviates colon lesions and regulates gut microbiota in diabetic mice. Scientific Reports, 13(1), 4897. https://doi.org/10.1038/s41598-023-31647-z
Huang, G., Xu, J., Lefever, D. E., Glenn, T. C., Nagy, T., & Guo, T. L. (2017). Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicology and Applied Pharmacology, 332, 138–148. https://doi.org/10.1016/j.taap.2017.04.009
Li, S., Zhou, L., Zhang, Q., Yu, M., & Xiao, X. (2022). Genistein improves glucose metabolism and promotes adipose tissue browning through modulating gut microbiota in mice. Food and Function, 13(22), 11715–11732. https://doi.org/10.1039/d2fo01973f
Yang, R., Jia, Q., Mehmood, S., Ma, S., & Liu, X. (2021). Genistein ameliorates inflammation and insulin resistance through mediation of gut microbiota composition in type 2 diabetic mice. European Journal of Nutrition, 60(4), 2155–2168. https://doi.org/10.1007/s00394-020-02403-0
Estruel-Amades, S., Massot-Cladera, M., Pérez-Cano, F. J., Franch, À., Castell, M., & Camps-Bossacoma, M. (2019). Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients, 11(2), 324. https://doi.org/10.3390/nu11020324
Li, J., Yuan, H., Zhao, Z., Li, L., Li, X., Zhu, L., Wang, X., Sun, P., & Xiao, Y. (2022). The mitigative effect of isorhamnetin against type 2 diabetes via gut microbiota regulation in mice. Frontiers in Nutrition, 9, 1070908. https://doi.org/10.3389/fnut.2022.1070908
Tian, B., Geng, Y., Wang, P., Cai, M., Neng, J., Hu, J., Xia, D., Cao, W., Yang, K., & Sun, P. (2022). Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. European Journal of Nutrition, 61(7), 3767–3783. https://doi.org/10.1007/s00394-022-02927-7
O’Keefe, S. J. (2008). Nutrition and colonic health: The critical role of the microbiota. Current Opinion in Gastroenterology, 24(1), 51–58. https://doi.org/10.1097/MOG.0b013e3282f323f3
Nie, Q., Hu, J., Gao, H., Li, M., Sun, Y., Chen, H., Zuo, S., Fang, Q., Huang, X., Yin, J., & Nie, S. (2021). Bioactive dietary fibers selectively promote gut microbiota to exert antidiabetic effects. Journal of Agricultural and Food Chemistry, 69(25), 7000–7015. https://doi.org/10.1021/acs.jafc.1c01465
Su, H., Xie, L., Xu, Y., Ke, H., Bao, T., Li, Y., & Chen, W. (2020). Pelargonidin-3-O-glucoside derived from wild raspberry exerts antihyperglycemic effect by inducing autophagy and modulating gut microbiota. Journal of Agricultural and Food Chemistry, 68(46), 13025–13037. https://doi.org/10.1021/acs.jafc.9b03338
Ling, Z., Liu, X., Jia, X., Cheng, Y., Luo, Y., Yuan, L., Wang, Y., Zhao, C., Guo, S., Li, L., Xu, X., & Xiang, C. (2014). Impacts of infection with different toxigenic Clostridium difficile strains on faecal microbiota in children. Scientific Reports, 4, 7485. https://doi.org/10.1038/srep07485
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., Sogin, M. L., Jones, W. J., Roe, B. A., Affourtit, J. P., Egholm, M., Henrissat, B., Heath, A. C., Knight, R., & Gordon, J. I. (2009). A core gut microbiome in obese and lean twins. Nature, 457(7228), 480–484. https://doi.org/10.1038/nature07540
Su, H., Mo, J., Ni, J., Ke, H., Bao, T., Xie, J., Xu, Y., Xie, L., & Chen, W. (2020). Andrographolide exerts antihyperglycemic effect through strengthening intestinal barrier function and increasing microbial composition of Akkermansia muciniphila. Oxidative Medicine and Cellular Longevity, 2020, 6538930. https://doi.org/10.1155/2020/6538930
Clemente, J. C., Ursell, L. K., Parfrey, L. W., & Knight, R. (2012). The impact of the gut microbiota on human health: An integrative view. Cell, 148(6), 1258–1270. https://doi.org/10.1016/j.cell.2012.01.035
Holmes, D. (2016). Gut microbiota: Antidiabetic drug treatment confounds gut dysbiosis associated with type 2 diabetes mellitus. Nature Reviews. Endocrinology, 12(2), 61. https://doi.org/10.1038/nrendo.2015.222
Chen, G., Ran, X., Li, B., Li, Y., He, D., Huang, B., Fu, S., Liu, J., & Wang, W. (2018). Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. eBioMedicine, 30, 317–325. https://doi.org/10.1016/j.ebiom.2018.03.030
Shi, X., Wei, X., Yin, X., Wang, Y., Zhang, M., Zhao, C., Zhao, H., McClain, C. J., Feng, W., & Zhang, X. (2015). Hepatic and fecal metabolomic analysis of the effects of Lactobacillus rhamnosus GG on alcoholic fatty liver disease in mice. Journal of Proteome Research, 14(2), 1174–1182. https://doi.org/10.1021/pr501121c
Sturm, A., & Dignass, A. U. (2008). Epithelial restitution and wound healing in inflammatory bowel disease. World Journal of Gastroenterology, 14(3), 348–353. https://doi.org/10.3748/wjg.14.348
Canfora, E. E., Jocken, J. W., & Blaak, E. E. (2015). Short-chain fatty acids in control of body weight and insulin sensitivity. Nature Reviews. Endocrinology, 11(10), 577–591. https://doi.org/10.1038/nrendo.2015.128
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vich Vila, A., Võsa, U., Mujagic, Z., Masclee, A. A. M., Jonkers, D. M. A. E., Oosting, M., Joosten, L. A. B., Netea, M. G., Franke, L., Zhernakova, A., Fu, J., Wijmenga, C., & McCarthy, M. I. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nature Genetics, 51(4), 600–605. https://doi.org/10.1038/s41588-019-0350-x
Tremaroli, V., & Bäckhed, F. (2012). Functional interactions between the gut microbiota and host metabolism. Nature, 489(7415), 242–249. https://doi.org/10.1038/nature11552
Jung, M. J., Lee, J., Shin, N. R., Kim, M. S., Hyun, D. W., Yun, J. H., Kim, P. S., Whon, T. W., & Bae, J. W. (2016). Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Scientific Reports, 6, 30887. https://doi.org/10.1038/srep30887
Vuillermin, P. J., O’Hely, M., Collier, F., Allen, K. J., Tang, M. L. K., Harrison, L. C., Carlin, J. B., Saffery, R., Ranganathan, S., Sly, P. D., Gray, L., Molloy, J., Pezic, A., Conlon, M., Topping, D., Nelson, K., Mackay, C. R., Macia, L., Koplin, J., Dawson, S. L., … BIS Investigator Group (2020). Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nature communications, 11(1), 1452https://doi.org/10.1038/s41467-020-14552-1
Verbrugghe, P., Brynjólfsson, J., Jing, X., Björck, I., Hållenius, F., & Nilsson, A. (2021). Evaluation of hypoglycemic effect, safety and immunomodulation of Prevotella copri in mice. Scientific Reports, 11(1), 21279. https://doi.org/10.1038/s41598-021-96161-6
Péan, N., Le Lay, A., Brial, F., Wasserscheid, J., Rouch, C., Vincent, M., Myridakis, A., Hedjazi, L., Dumas, M. E., Grundberg, E., Lathrop, M., Magnan, C., Dewar, K., & Gauguier, D. (2020). Dominant gut Prevotella copri in gastrectomised non-obese diabetic Goto-Kakizaki rats improves glucose homeostasis through enhanced FXR signalling. Diabetologia, 63(6), 1223–1235. https://doi.org/10.1007/s00125-020-05122-7
Gorvitovskaia, A., Holmes, S. P., & Huse, S. M. (2016). Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome, 4, 15. https://doi.org/10.1186/s40168-016-0160-7
Daniel, H., Gholami, A. M., Berry, D., Desmarchelier, C., Hahne, H., Loh, G., Mondot, S., Lepage, P., Rothballer, M., Walker, A., Böhm, C., Wenning, M., Wagner, M., Blaut, M., Schmitt-Kopplin, P., Kuster, B., Haller, D., & Clavel, T. (2014). High-fat diet alters gut microbiota physiology in mice. The ISME Journal, 8(2), 295–308. https://doi.org/10.1038/ismej.2013.155
Liu, S., Qin, P., & Wang, J. (2019). High-fat diet alters the intestinal microbiota in streptozotocin-induced type 2 diabetic mice. Microorganisms, 7(6), 176. https://doi.org/10.3390/microorganisms7060176
Zhang, C., Li, S., Yang, L., Huang, P., Li, W., Wang, S., Zhao, G., Zhang, M., Pang, X., Yan, Z., Liu, Y., & Zhao, L. (2013). Structural modulation of gut microbiota in life-long calorie-restricted mice. Nature Communications, 4, 2163. https://doi.org/10.1038/ncomms3163
Park, S. K., Kim, M. S., & Bae, J. W. (2013). Blautia faecis sp. nov., isolated from human faeces. International Journal of Systematic and Evolutionary Microbiology, 63(pt 2), 599–603. https://doi.org/10.1099/ijs.0.036541-0
Ji, Y., Yin, Y., Li, Z., & Zhang, W. (2019). Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD). Nutrients, 11(8), 1712. https://doi.org/10.3390/nu11081712
Tian, Y., Cai, J., Gui, W., Nichols, R. G., Koo, I., Zhang, J., Anitha, M., & Patterson, A. D. (2019). Berberine directly affects the gut microbiota to promote intestinal Farnesoid X receptor activation. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 47(2), 86–93. https://doi.org/10.1124/dmd.118.083691
Zhang, Y., Gu, Y., Ren, H., Wang, S., Zhong, H., Zhao, X., Ma, J., Gu, X., Xue, Y., Huang, S., Yang, J., Chen, L., Chen, G., Qu, S., Liang, J., Qin, L., Huang, Q., Peng, Y., Li, Q., Wang, X., … Wang, W. (2020). Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nature communications, 11(1), 5015. https://doi.org/10.1038/s41467-020-18414-8
Rios-Covian, D., Gueimonde, M., Duncan, S. H., Flint, H. J., & de Reyes-Gavilanlos, C. G. (2015). Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiology Letters, 362(21), fnv176. https://doi.org/10.1093/femsle/fnv176
Sun, L., Xie, C., Wang, G., Wu, Y., Wu, Q., Wang, X., Liu, J., Deng, Y., Xia, J., Chen, B., Zhang, S., Yun, C., Lian, G., Zhang, X., Zhang, H., Bisson, W. H., Shi, J., Gao, X., Ge, P., Liu, C., … Jiang, C. (2018). Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nature medicine, 24(12), 1919–1929. https://doi.org/10.1038/s41591-018-0222-4
Gu, Y., Wang, X., Li, J., Zhang, Y., Zhong, H., Liu, R., Zhang, D., Feng, Q., Xie, X., Hong, J., Ren, H., Liu, W., Ma, J., Su, Q., Zhang, H., Yang, J., Wang, X., Zhao, X., Gu, W., Bi, Y., … Wang, W. (2017). Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nature communications, 8(1), 1785. https://doi.org/10.1038/s41467-017-01682-2
Staley, C., Weingarden, A. R., Khoruts, A., & Sadowsky, M. J. (2017). Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Applied Microbiology and Biotechnology, 101(1), 47–64. https://doi.org/10.1007/s00253-016-8006-6
Sun, R., Yang, N., Kong, B., Cao, B., Feng, D., Yu, X., Ge, C., Huang, J., Shen, J., Wang, P., Feng, S., Fei, F., Guo, J., He, J., Aa, N., Chen, Q., Pan, Y., Schumacher, J. D., Yang, C. S., Guo, G. L., … Wang, G. (2017). Orally administered berberine modulates hepatic lipid metabolism by altering microbial bile acid metabolism and the intestinal FXR signaling pathway. Molecular pharmacology, 91(2), 110–122. https://doi.org/10.1124/mol.116.106617
Centanni, M., Lawley, B., Butts, C. A., Roy, N. C., Lee, J., Kelly, W. J., & Tannock, G. W. (2018). Bifidobacterium pseudolongum in the Ceca of rats fed hi-maize starch has characteristics of a keystone species in bifidobacterial blooms. Applied and Environmental Microbiology, 84(15), e00547-e618. https://doi.org/10.1128/AEM.00547-18
Acknowledgements
The author is thankful to the Indian Institute of Technology (BHU) and MHRD, India, for fellowship. The authors thankfully acknowledge to Prof. Sushant Kumar Shrivastava formal Head of Department, Pharmaceutical Engineering and Technology, IIT (BHU) Varanasi-221 005, Uttar Pradesh, India, for guidance during the work. The author is also thankful to SATHI BHU for the High-Resolution Accurate Mass Spectrometry (HRMS) study for plant metabolomics.
Author information
Authors and Affiliations
Contributions
Amit Kumar Singh: conceptualization, methodology, writing-original draft preparation. Pradeep Kumar: methodology, writing—original draft preparation. Vishnu D Rajput: conceptualization, formal analysis, validation. Sunil Kumar Mishra: conceptualization, methodology, formal analysis, supervision. Kavindra Nath Tiwari: conceptualization, formal analysis, validation. Anand Kumar Singh: formal analysis, investigation, writing—review and editing. Tatiana Minkina: formal analysis, writing—review and editing, validation. Ajay Kumar Pandey: formal analysis, validation. Prabhat Upadhyay: formal analysis, validation. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All of the tests were carried out in compliance with Institutional animal experimentation rules. The Central Animal Ethics Committee, as per CPCSEA guidelines at IMS BHU Varanasi 221005 (Reg. No. 542/GO/Rebi//S/02/CPCSEA dated 26.5.2017), approved this study (Approval number: Dean/2021/IAEC/2560). The CPCSEA guidelines were fully followed in the experiment.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, A.K., Kumar, P., Mishra, S.K. et al. A Dual Therapeutic Approach to Diabetes Mellitus via Bioactive Phytochemicals Found in a Poly Herbal Extract by Restoration of Favorable Gut Flora and Related Short-Chain Fatty Acids. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-04879-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-04879-6