Abstract
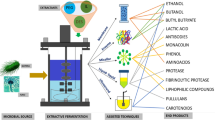
In the current scenario, where environmental degradation, global climate change, and the depletion of petroleum feedstock pose significant challenges, the chemical industry seeks sustainable alternatives for manufacturing chemicals, fuels, and bioplastics. Biorefining processes that integrate biomass conversion and microbial fermentation have emerged as preferred approaches to create value-added compounds. However, commercializing biorefinery products is hindered by dilute concentrations of final products and the demand for high purity goods. To address these challenges, effective separation and recovery procedures are essential to minimize costs and equipment size. This article proposes a biorefinery route for the production of protocatechuic acid (PCA) by focusing on in situ PCA separation and purification from fermentation broth. PCA is a significant phenolic molecule with numerous applications in the pharmaceutical sector for its anti-inflammatory, antiapoptotic, and antioxidant properties, as well as in the food, polymer, and other chemical industries. The chemical approach is predominantly used to produce PCA due to the cost-prohibitive nature of natural extraction techniques. Reactive extraction, a promising technique known for its enhanced extraction efficiency, is identified as a viable strategy for recovering carboxylic acids compared to conventional methods. The extraction of PCA has been explored using various solvents, including natural and conventional solvents, such as aminic and organophosphorous extractants, as well as the potential utilization of ionic liquids as green solvents. Additionally, back extraction techniques like temperature swing and diluent composition swing can be employed for reactive extraction product recovery, facilitating the regeneration of the extractant from the organic phase. By addressing the challenges associated with PCA production and usage, particularly through reactive extraction, this proposed biorefinery route aims to contribute to a more sustainable and environmentally friendly chemical industry. The incorporation of PCA in the biorefinery process allows for the utilization of this valuable compound with diverse industrial applications, thus providing an additional incentive for the development and optimization of efficient separation techniques.




Similar content being viewed by others
Data availability
This is not applicable.
References
Biddy, M. J., Scarlata, C., & Kinchin, C. (2016). Chemicals from biomass: a market assessment of bioproducts with near-term potential (No. NREL/TP-5100-65509). National Renewable Energy Lab. (NREL).
Spaeth J. (2014). “Country report United States.” http://www.iea-bioenergy.task42- biorefineries.com/en/ieabiorefinery/Show-9/Country-report-United-States-2014-available.htm.
Fawzy, S., Osman, A. I., Doran, J., & Rooney, D. W. (2020). Strategies for mitigation of climate change: A review. Environmental Chemistry Letters, 18, 2069–2094.
Food and Agriculture Organization of the United Nations. (2015). Food wastage footprint & climate change.
Ozturk, B., Winterburn, J., & Gonzalez-Miquel, M. (2019). Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochemical Engineering Journal, 151, 107298.
Food and Agriculture Organization of the United Nations (FAO). (2019). State of food and agriculture. In Moving forward on food loss and waste production.
WEF. (2010). The future of industrial biorefineries. World Economic Forum Available from: http://www3.weforum.org/docs/WEF_FutureIndustrialBiorefineries_Report_2010.pdf
Cherubini, F., Jungmeier, G., Wellisch, M., Willke, T., Skiadas, I., Van Ree, R., & De Jong, E. (2009). Toward a common classification approach for biorefinery systems. Biofuels, Bioproducts and Biorefining, 3, 534–546.
Jungmeier G, Cherubini F, Dohy M, de Jong E, Jørgensen H, Mandl M, Willke T. (2009). Definition and classification of biorefinery systems? The approach in IEA Bioenergy Task 42 biorefineries. Presentation held at the biorefinery course adding value to the sustainable utilisation of biomass. .
Riemenschneider, W., & Tanifuji, M. (2000). Carboxylic acids, aliphatic. Ullmann's Encyclopedia of Industrial Chemistry, 99–111.
Worrell E; Phylipsen D, Einstein D, Martin N. (2000) Energy use and energy intensity of the US Chemical Industry. US DOE Report LBNL-44314. Available online: http://ateam.lbl.gov/PUBS/doc/LBNL44314.pdf (accessed on 8 November 2016)
Werpy, T., & Petersen, G. (2004). Top value added chemicals from biomass: volume I--results of screening for potential candidates from sugars and synthesis gas (No. DOE/GO-02004-1992). Golden, CO (United States): National Renewable Energy Lab. (NREL).
Liao, J. C., Mi, L., Pontrelli, S., & Luo, S. (2016). Fuelling the future: microbial engineering for the production of sustainable biofuels. Nature Reviews. Microbiology, 14(5), 288–304.
Murali, N., Fernandez, S., & Ahring, B. K. (2017). Fermentation of wet-exploded corn stover for the production of volatile fatty acids. Bioresource Technology, 227, 197–204.
Honda, H., Toyama, Y., Takahashi, H., Nakazeko, T., & Kobayashi, T. (1995). Effective lactic acid production by two-stage extractive fermentation. Journal of Fermentation and Bioengineering, 79, 589–593.
Evans, P. J., & Wang, H. Y. (1990). Effects of extractive fermentation on butyric acid production by Clostridium acetobutylicum. Applied Microbiology and Biotechnology, 32, 393–397.
Hatzinikolaou, D. G., & Wang, H. Y. (1992). Extractive fermentation systems for organic acids production. Canadian Journal of Chemical Engineering, 70, 543–552.
Yabannavar, V. M., & Wang, D. I. C. (1991). Strategies for reducing solvent toxicity in extractive fermentations. Biotechnology and Bioengineering, 37, 716–722.
Atkinson, B., & Mavituna, F. (1983). Biochemical engineering and biotechnology handbook. The Nature Press-MacMillan.
Boyaval, P., Corre, C., & Terre, S. (1987). Continuous lactic acid fermentation with concentrated product recovery by ultra-filtration and electrodialysis. Biotechnology Letters, 9(3), 207–212.
Hauer, E., & Marr, R. (1994). Liquid extraction in biotechnology. International Journal of Chemical Engineering, 34(2), 178–187.
Wardell, J. M., & King, C. J. (1978). Solvent equilibria for extraction of carboxylic acids from water. Journal of Chemical & Engineering Data, 23, 144–148.
Timmer, J. K. M., Kromkamp, J., & Robbertsen, T. (1994). Lactic acid separation from fermentation broth by reverse osmosis and nanofiltration. Journal of Membrane Science, 92, 185–197.
Cockrem M C M, Johnson P D, (1991) Recovery of lactate and lactic acid from fermentation broth. U.S. Patent 5,210,296.
Hongo, M., Nomura, Y., & Iwahara, M. (1986). Novel method of lactic acid production by electrodialysis fermentation Appl. Environmental Microbiology, 52(2), 314.
Sirman, T., Pyle, D. L., & Grandison, A. S. (1991). Extraction of organic acids using a supported liquid membrane. Biochemical Society Transactions, 19(3), 274–279.
Pazouki, M., & Panda, T. (1998). Recovery of citric acid - A review. Bioprocess Engineering, 19, 435–439.
Cao, X., Yun, H. S., & Koo, Y. M. (2002). Recovery of lactic acid by anion-exchange resin Amberlite IRA-400. Biochemical Engineering Journal, 11, 189–196.
Kertes, A. S., & King, C. J. (1986). Extraction chemistry of fermentation product carboxylic acids. Biotechnology and Bioengineering, 28(2), 269–282.
Polyphenols market size, share & trends analysis report by product (grape seed, green tea, cocoa), by application (beverages, food, feed, dietary supplements, cosmetics), and segment forecasts, 2019–2025. Available from: https://www.grandviewresearch.com/industry-analysis/polyphenols-market-analysis (2019). Accessed Nov 2022.
Herrmann, K. (1989). Ocurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Critical Reviews in Food Science & Nutrition, 28, 315–347.
Kayano, S., Kikuzaki, H., Fukutsuka, N., Mitani, T., & Nakatani, N. (2002). Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. Journal of Agricultural and Food Chemistry, 50, 3708–3712.
Li, H., Hu, X., Zhang, Y., et al. (2015). High-capacity magnetic hollow porous molecularly imprinted polymers for specific extraction of protocatechuic acid. Journal of Chromatography A, 1404, 21–27. https://doi.org/10.1016/j.chroma.2015.05.038
Huang, W. Y., Zhang, H. C., Liu, W. X., & Li, C. Y. (2012). Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. Journal of Zhejiang University. Science. B, 13, 94–102.
Palafox-Carlos, H., Gil-Chavez, J., Sotelo-Mundo, R. R., Namiesnik, J., Gorinstein, S., & Gonzalez-Aguilar, G. A. (2012). Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’ mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules, 17, 12657–12664.
Masella, R., Santangelo, C., D’Archivio, M., Li Volti, G., Giovannini, C., & Galvano, F. (2012). Protocatechuic acid and human disease prevention: Biological activities and molecular mechanisms. Current Medicinal Chemistry, 19, 2901–2917.
Ellnain-Wojtaszek, M. (1997). Phenolic acids from Ginkgo biloba L. Part II. Quantitative analysis of free and liberated by hydrolysis phenolic acids. Acta Poloniae Pharmaceutica, 54, 229–232.
Ali, B. H., Al Wabel, N., & Blunden, G. (2005). Phytochemical, pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A review. Phytotherapy Research, 19, 369–375.
Jurgenliemk, G., & Nahrstedt, A. (2002). Phenolic compounds from Hypericum perforatum. Planta Medica, 68, 88–91.
Juurlink, B. H., Azouz, H. J., Aldalati, A. M., AlTinawi, B. M., & Ganguly, P. (2014). Hydroxybenzoic acid isomers and the cardiovascular system. Nutrition Journal, 13(1), 1–10.
de Ferrars, R. M., Czank, C., Zhang, Q., Botting, N. P., Kroon, P. A., Cassidy, A., & Kay, C. D. (2014). The pharmacokinetics of anthocyanins and their metabolites in humans. British Journal of Pharmacology, 171, 3268–3282. https://doi.org/10.1111/bph.12676
Czank, C., Cassidy, A., Zhang, Q., Morrison, D. J., Preston, T., Kroon, P. A., Botting, N. P., & Kay, C. D. (2013). Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. The American Journal of Clinical Nutrition, 97, 995–1003.
Yang, Y. C., Wei, M. C., Lian, F. Y., & Huang, T. C. (2013). Simultaneous extraction and quantitation of oleanolic acid and ursolic acid from Scutellariabarbata D. Don by ultrasound-assisted extraction and high-performance liquid chromatography. Chemical Engineering Communications, 201, 482–500.
Link, K. P., Angell, H. R., & Walker, J. C. (1929). The isolation of protocatechuic acid from pigmented onion scales and its significance in relation to disease resistance in onions. The Journal of Biological Chemistry, 81(2), 369–375.
Stanier, R. Y., & Ingraham, J. L. (1954). Protocatechuic acid oxidase. Journal of Biological Chemistry, 210(2), 799–808.
Guenzi, W. D., & McCalla, T. M. (1966). Phytotoxic substances extracted from soil. Soil Science Society of America Journal, 30(2), 214–216.
Irwin, B. Y., & Pearl, A. (1946). Reactions of vaillin and its derived compounds. The Caustic fusion of vaillin. Journal of the American Chemical Society, 68, 2180–2184.
Priefert, H., Rabenhorst, J., & Steinbuchel, A. (1997). Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. Journal of Bacteriology, 179, 2595–2607.
Chaabane Elaoud, S., Abdelhedi, R., & Savall, A. (2001). Oxydation électrochimique de l’acide vanillique sur des oxydes d’or et de plomb. Journal-Societe Chimique De Tunisie, 4, 1029–1042.
Guo, X., Wang, X., Chen, T., et al. (2020). Comparing E. coli monocultures and co-cultures for biosynthesis of protocatechuic acid and hydroquinone. Biochemical Engineering Journal, 156, 107518. https://doi.org/10.1016/j.bej.2020.107518
Wilson, M. K., Abergel, R. J., Raymond, K. N., Arceneaux, J. E. L., & Byers, B. R. (2006). Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis Biochem. Biochemical and Biophysical Research Communications, 348, 320–325.
Garner, B. L., Arceneaux, J. E., & Byers, B. R. (2004). Temperature control of a 3,4-dihydroxybenzoate (protocatechuate)-based siderophore in Bacillus anthracis. Current Microbiology, 49, 89–94.
Williams, K. M., Martin, W. E., Smith, J., Williams, B. S., & Garner, B. L. (2012). Production of protocatechuic acid in Bacillus thuringiensis ATCC33679. International Journal of Molecular Sciences, 13, 3765–3772.
Okai, N., Miyoshi, T., Takeshima, Y., Kuwahara, H., Ogino, C., & Kondo, A. (2016). Production of protocatechuic acid by Corynebacterium glutamicum expressing chorismate-pyruvate lyase from Escherichia coli. Applied Microbiology and Biotechnology, 100, 135–145.
Lubbers, R. J. M., & de Vries, R. P. (2021). Production of protocatechuic acid from phydroxyphenyl (H) units and related aromatic compounds using an Aspergillus niger cell factory. mBio, 12, e00391–e00321. https://doi.org/10.1128/mBio.00391-21
Rekik, R., Hamza, M., Jaziri, M., & Abdelhedi, R. (2020). Electrochemical oxidation of vanillic acid by electro-Fenton process: Toward a novel route of protocatechuic acid electrosynthesis. Arabian Journal of Chemistry, 13(1), 357–365.
Lubbers, R. J. M., Dilokpimol, A., Visser, J., Mäkelä, M. R., Hildén, K. S., & de Vries, R. P. (2019). A comparison between the homocyclic aromatic metabolic pathways from plant-derived compounds by bacteria and fungi. Biotechnology Advances, 37, 107396. https://doi.org/10.1016/j.biotechadv.2019.05.002
Boschloo, J. G., Moonen, E., van Gorcom, R. F. M., Hermes, H. F. M., & Bos, C. J. (1991). Genetic analysis of Aspergillus niger mutants defective in benzoate-4- hydroxylase function. Current Genetics, 19, 261–264. https://doi.org/10.1007/BF00355052
Homma, T., Tsurusaki, Y., Kamimura, N., Masai, E., & Ang, L. Z. P. (2021). Protocatechuic acid fuel cell: A sustainable energy generation system based on microbial metabolism of lignin-derived aromatic compounds. Biomass & Bioenergy, 154, 106254.
Johnson, C., Salvachua, D., Khanna, P., Smith, H., Peterson, D., & Beckham, G. (2016). Enhancing muconic acid production from glucose and lignin-derived aromatic compounds via increased protocatechuate decarboxylase activity. Metabolic Engineering Communications, 3, 111–119. https://doi.org/10.1016/j.meteno.2016.04.002
Semaming, Y., Pannengpetch, P., Chattipakorn, S. C., & Chattipakorn, N. (2015). Pharmacological properties of protocatechuic Acid and its potential roles as complementary medicine. Evidence-based Complementary and Alternative Medicine, 2015, 593902. https://doi.org/10.1155/2015/593902
Song, J., He, Y., Luo, C., Feng, B., Ran, F., Xu, H., & Zhang, D. (2020). New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacological Research, 161, 105109. https://doi.org/10.1016/j.phrs.2020.105109
Zhong, C., Hou, P. F., Li, Y. X., Yang, W. Y., Shu, M., & Wu, G. P. (2021). Characterization, antioxidant and antibacterial activities of gelatin film incorporated with protocatechuic acid and its application on beef preservation. LWT, 151, 112154. https://doi.org/10.1016/j.lwt.2021.112154
Liu, J., Liu, S., Wu, Q. Q., Gu, Y. Y., Kan, J., & Jin, C. H. (2017). Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocolloids, 73, 90–100. https://doi.org/10.1016/j.foodhyd.2017.06.035
Tanaka, T., Kawamori, T., Ohnishi, M., Okamoto, K., Mori, H., & Hara, A. (1994). Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis by dietary protocatechuic acid during initiation and postinitiation phases. Cancer Research, 54, 2359–2365.
Simić, A., Manojlović, D., Šegan, D., & Todorović, M. (2007). Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules, 12, 2327–2340.
Bock, L. H., & Anderson, J. K. (1958). Linear polyesters derived from protocatechuic acid. Journal of Polymer Science, 28(116), 121–127. https://doi.org/10.1002/pol.1958.1202811611
Sun, J. J., Zhou, D. M., Fang, H. Q., & Chen, H. Y. (1998). The electrochemical copolymerization of 3, 4-dihydroxybenzoic acid and aniline at microdisk gold electrode and its amperometric determination for ascorbic acid. Talanta, 45(5), 851–856.
Qin, Y., Song, F., Ai, Z., Zhang, P., & Zhang, L. (2015). Protocatechuic acid promoted alachlor degradation in Fe(III)/H2O2 Fenton system. Environmental Science & Technology, 49(13), 7948–7956. https://doi.org/10.1021/es506110w
Whittle, N., Eldridge, H., & Bartley, J. (1999). Identification of polyphenols in barley and beer by HPLC/MS and HPLC/electrochemical detection. Journal of the Institute of Brewing, 105, 89–99.
Janer del VaUe, L. (1980). Contaminaci6n de las aguas por el alpechln y posibles soluciones al problema. Grasas y Aceites, 31(4), 273–279.
Peters, R. W., Walker, T. J., Ku, Y., Berdanier, B., Chang, T. K., & Freund, D. (1983). Wastewater treatment. Physical and chemical methods. Journal (Water Pollution Control Federation), 55(6), 599–512.
Benitez, J. F., Beltran-Heredia, J., & Acero, J. L. (1993). Protocatechuic acid ozonation in aqueous solutions. Water Research, 27(10), 1519–1525. https://doi.org/10.1016/0043-1354(93)90096-z
Gernjak, W., Krutzler, T., Glaser, A., Malato, S., Caceres, J., Bauer, R., & Fernandez-Alba, A. R. (2003). Photo-Fenton treatment of water containing natural phenolic pollutants. Chemosphere, 50, 71–78.
Benitez, F. J., Beltran-Heredia, J., Acero, J. L., & Gonzalez, T. (1996s). Degradation of protocatechuic acid by two advanced oxidation processes: Ozone/UV radiation and H2O2/UV radiation. Water Research, 30, 1597–1604.
Rivas, F. J., Frades, J., Alonso, M. A., Montoya, C., & Monteagudo, J. M. (2005). Fenton’s oxidation of food processing wastewater components. Kinetic modeling of protocatechuic acid degradation. Journal of Agricultural and Food Chemistry, 53(26), 10097–10104. https://doi.org/10.1021/jf0512712
de Heredia, A. J. B., Antón, J. T., Rodríguez, J. G., Viseas, M. D. P. R., & Vargas, J. R. D. (2000). Aerobic biological treatment of olive mill wastewater previously treated by an ozonation stage. Grasas y Aceites, 51(5), 332–339.
Regulation (EC). No 1333/2008 of the European Parliament and of the Council of 16 On food additives (December 2008). The Official Journal of the European Union, 354, 16–33.
EFSA. (2012). Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific opinion on the reevaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA Journal, 10(3), 2588.
Cruz, J. M., Moldes, A. B., Bustos, G., Torrado, A., & Domínguez, J. M. (2007). Integral utilization of barley husk for the production of food additives. Journal of the Science of Food and Agriculture, 87, 1000–1008.
Barbosa-Pereira, L., Bilbao, A., Vilches, P., Angulo, I., & LLuis J, Fité B, Cruz J M. (2014). Brewery waste as a potential source of phenolic compounds: Optimisation of the extraction process and evaluation of antioxidant and antimicrobial activities. Food Chemistry, 145, 191–197.
Sedej, I., Milczarek, R., Wang, S. C., Sheng, R., de Jesús, A.-B. R., Dao, L., & Takeoka, G. (2016). Spray drying of a phenolic-rich membrane filtration fraction of olive mill wastewater: optimisation and dried product quality. International Journal of Food Science, 51(8), 1900–1909. https://doi.org/10.1111/ijfs.13163
Russo, C. (2007). A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). Journal of Membrane Science, 288, 239–246.
Sarma, J., & Mahiuddin, S. (2014). Specific ion effect on the point of zero charge of α-alumina and on the adsorption of 3, 4-dihydroxybenzoic acid onto α-alumina surface. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 457, 419–424.
Demir, Ö., Gök, A., & Kırbaşlar, Ş. İ. (2022). Optimization of protocatechuic acid adsorption onto weak basic anion exchange resins: Kinetic, mass transfer, isotherm, and thermodynamic study. Biomass Conversion and Biorefinery, 1–17.
Kelly, N. P., Kelly, A. L., & O'Mahony, J. A. (2019). Strategies for enrichment and purification of polyphenols from fruit-based materials. Trends in Food Science & Technology, 83, 248–258. https://doi.org/10.1016/J.TIFS.2018.11.010
Porgali, E., & Büyüktuncel, E. (2012). Determination of phenolic composition and antioxidant capacity of native red wines by high performance liquid chromatography and spectrophotometric methods. Food Research International, 45, 145–154.
Cañadas, R., Díaz, I., Rodríguez, M., González, E. J., & González-Miquel, M. (2022). An integrated approach for sustainable valorization of winery wastewater using bio-based solvents for recovery of natural antioxidants. Journal of Cleaner Production, 334, 130181.
Huang, H., Yang, S., & Ramey, D. E. (2004). Applied Biochemistry and Biotechnology, 114(1–3), 671.
Antony, F. M., & Wasewar, K. (2018). Separation of protocatechuic acid using di-(2-ethylhexyl) phosphoric acid in isobutyl acetate, toluene, and petroleum ether. Journal of Chemical & Engineering Data, 63(3), 587–597.
Antony, F. M., & Wasewar, K. (2020). Effect of temperature on equilibria for physical and reactive extraction of protocatechuic acid. Heliyon, 6(5), e03664.
Antony, F. M., & Wasewar, K. L. (2018). Reactive separation of protocatechuic acid using tri-n-octyl amine and di-(2-ethylhexyl) phosphoric acid in methyl isobutyl ketone. Separation and Purification Technology, 207, 99–107.
Antony, F. M., & Wasewar, K. L. (2022). Experimental investigation on recovery of bio-based protocatechuic acid using ionic liquids. Journal of Chemical Technology and Biotechnology, 97(11), 3144–3151.
Antony, F. M., Wasewar, K. L., De, B. S., & Kumar, S. (2019). Separation of protocatechuic acid using tri-n-octylamine: experimental and mathematical investigation. Journal of Chemical & Engineering Data, 64(3), 1101–1112.
Antony, F. M., Wasewar, K., & De, B. S. (2019). Efficacy of tri-n-octylamine, tri-n-butyl phosphate and di-(2-ethylhexyl) phosphoric acid for reactive separation of protocatechuic acid. Separation Science and Technology, 54(18), 3100–3114.
De, B. S., Wasewar, K. L., & Dhongde, V. R. (2018). Extractive separation of protocatechuic acid using natural non-toxic solvents and conventional solvents. Chemical Data Collections, 15–16, 244–253.
De, B. S., Wasewar, K. L., Dhongde, V. R., Ingle, A. A., & Mondal, H. (2018). Experimental and modeling of reactive separation of protocatechuic acid. Chemical Engineering Research and Design, 132, 593–605.
Demir, Ö., İsayev, İ., Gök, A., & Kırbaşlar, Ş. İ. (2023). The application of Box–Behnken design for the optimization of protocatechuic acid separation by a reactive extractant trioctylphosphine oxide. Biomass Conversion and Biorefinery, 1–13.
Antony, F. M., & Wasewar, K. L. (2023). Ionic liquids as green solvents in process industry for reaction and separation: Emphasizing on protocatechuic acid recovery. Chemical Engineering Communications, 1–8.
Bizek, A., Honacek, J., Rericha, R., & Kousova, M. (1992). Amine extraction of hydrocarboxylic acids. 1.Extraction of citric acid with 1-octanol/n-heptane solutions of trialkylamine. Industrial and Engineering Chemistry Research, 31, 1554–1562.
Eyal, A. M., & Canari, R. (1995). pH dependence of carboxylic and mineral acid extraction by amine-based extractants: Effects of pKa, amine basicity, and diluent properties. Industrial & Engineering Chemistry Research, 34(5), 1789–1798.
Schlosser, Š., Marták, J., & Blahušiak, M. (2018). Specific phenomena in carboxylic acids extraction by selected types of hydrophobic ionic liquids. Chemical Papers, 72, 567–584.
Marták, J., & Schlosser, Š. (2007). Extraction of lactic acid by phosphonium ionic liquids. Separation and Purification Technology, 57(3), 483–494.
Solichien, M. S., O'Brien, D., Hammond, E. G., & Glatz, C. E. (1995). Membrane-based extractive fermentation to produce propionic and acetic acids: Toxicity and mass transfer considerations. Enzyme and Microbial Technology, 17(1), 23–31.
Laane, C., Boeren, S., & Vos, C. (1985). On the optimization of organic solvents in multi-liquid-phase biocatalysis. Trends in Biotechnology, 3(10), 251–252.
Roffler, S. R., Randolph, T. W., Miller, D. A., Blanch, H. W., & Prausnitz, J. M. (2021). Extractive bioconversions with nonaqueous solvents. In Extractive bioconversions (pp. 133–172). CRC Press.
Brink, L. E. S., & Tramper, J. (1985). Optimization of organic solvent in multiphase biocatalysis. Biotechnology and Bioengineering, 27(8), 1258–1269.
Matsumura, M., & Märkl, H. (1986). Elimination of ethanol inhibition by perstraction. Biotechnology and Bioengineering, 28(4), 534–541.
Wood, N., Ferguson, J. L., Gunaratne, H. N., Seddon, K. R., Goodacre, R., & Stephens, G. M. (2011). Screening ionic liquids for use in biotransformations with whole microbial cells. Green Chemistry, 13(7), 1843–1851.
Rockman, J. T., Kehat, E., & Lavie, R. (1997). Thermally enhanced extraction of citric acid through supported liquid membrane. AICHE Journal, 43, 2376–2380.
Baniel, A. M., A. M. Eyal, and K. Hajdu (1981) Recovery of acids from aqueous solutions. U.S. Patent 4,275,234.
Tamada, J. A., & King, C. J. (1990). Extraction of carboxylic acids with amine extractants. 3. Effect of temperature, water coextraction, and process considerations. Industrial & Engineering Chemistry Research, 29, 1333–1338.
Yabannavar, V. M., & Wang, D. I. C. (1991). Extractive fermentation for lactic acid production. Biotechnology and Bioengineering, 37, 1095–1100.
Poole, L. J., & King, C. J. (1991). Regeneration of carboxylic acid-amine extracts by back-extraction with an aqueous solution of a volatile amine. Industrial & Engineering Chemistry Research, 30, 923–929.
King, C. J. (1992). Amine-based systems for carboxylic acid recovery. CHEMTECH, 285–291.
Hong, Y. K., Hong, W. H., & Han, D. H. (2001). Application of reactive extraction to recovery of carboxylic acids. Biotechnology and Bioprocess Engineering, 6(6), 386–394.
Keshav, A., & Wasewar, K. L. (2010). Back extraction of propionic acid from loaded organic phase. Chemical Engineering Science, 65(9), 2751–2757.
Author information
Authors and Affiliations
Contributions
Antony FA: conceptualization, experimentation, writing—original draft, data curation, investigation, and interpretation of data. Wasewar KL: conceptualization, supervision, data analysis, writing and language editing of the manuscript, and communication.
Corresponding author
Ethics declarations
Ethical Approval
This is not applicable.
Consent to Participate
This is not applicable.
Consent to Publish
This is not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Antony, F.M., Wasewar, K.L. The Sustainable Approach of Process Intensification in Biorefinery Through Reactive Extraction Coupled with Regeneration for Recovery of Protocatechuic Acid. Appl Biochem Biotechnol 196, 1570–1591 (2024). https://doi.org/10.1007/s12010-023-04659-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04659-8