Abstract
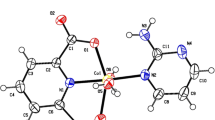
In this work, we aimed to synthesize a new cobalt(II) complex, namely [Co2(μ-HIPA)(NC)2(H2O)3(NO3)]·(NO3)(C2H5OH)(1) (where H3IPA = 5-hydroxy isophthalic acid and NC = 2,9-dimethyl-1,10-phenanthroline or neocuproine), as a promising chemotherapeutic agent. The diffraction (single crystal-XRD and powder-XRD), spectroscopic (FTIR and UV–visible), molar conductance, and thermal techniques were used to characterize complex 1. Single-crystal X-ray diffraction analysis reveals that Co(II) exists in an octahedral geometry, with the ligation of four oxygen atoms, and two nitrogen atoms. Topological analysis of complex 1 reveals 2,6C6 topological type as an underlying net. The plausible intermolecular interactions within complex 1 that control the crystal packing were analyzed by Hirshfeld surface analysis. In vitro cytotoxicity of complex 1 was evaluated against acute myeloid leukemia (THP-1), colorectal (SW480), and prostate (PC-3) cancer cell lines by utilizing an MTT assay. The result shows that complex 1 can inhibit the growth of cancer cells (THP-1, SW480, and PC-3) at lower inhibitory concentration (IC50) values of > 100, 43.6, and 95.1 µM respectively. The morphological changes induced by complex 1 on THP-1 and SW480 cancer cell lines were carried out with acridine orange/ethidium bromide staining methods. Additionally, comprehensive molecular docking studies were performed to understand the potential binding interactions of complex 1 with different bio-macromolecules.








Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Lalia-Kantouri, M., Gdaniec, M., Choli-Papadopoulou, T., Badounas, A., Papadopoulos, C. D., Czapik, A., & Tsitouroudi, F. (2012). Effect of cobalt(II) complexes with dipyridylamine and salicylaldehydes on cultured tumor and non-tumor cells: Synthesis, crystal structure investigations and biological activity. Journal of Inorganic Biochemistry, 117, 25–34. https://doi.org/10.1016/J.JINORGBIO.2012.08.022
Alvarez, N., Viña, D., Leite, C. M., Mendes, L. F. S., Batista, A. A., Ellena, J., Facchin, G. (2020). Synthesis and structural characterization of a series of ternary copper(II)-L-dipeptide-neocuproine complexes. Study of their cytotoxicity against cancer cells including MDA-MB-231, triple negative breast cancer cells. Journal of Inorganic Biochemistry, 203, 110930. https://doi.org/10.1016/J.JINORGBIO.2019.110930
Laws, K., & Suntharalingam, K. (2018). The next generation of anticancer metallopharmaceuticals: Cancer stem cell-active inorganics. ChemBioChem, 19(21), 2246–2253. https://doi.org/10.1002/CBIC.201800358
Gourdon, L., Cariou, K., & Gasser, G. (2022). Phototherapeutic anticancer strategies with first-row transition metal complexes: A critical review. Chemical Society Reviews, 51(3), 1167–1195. https://doi.org/10.1039/D1CS00609F
Geersing, A., Ségaud, N., van der Wijst, M. G. P., Rots, M. G., & Roelfes, G. (2018). Importance of metal-ion exchange for the biological activity of coordination complexes of the biomimetic ligand N4Py. Inorganic Chemistry, 57(13), 7748–7756. https://doi.org/10.1021/acs.inorgchem.8b00714
Amiri, N., Nouir, S., Hajji, M., Roisnel, T., Guerfel, T., Simonneaux, G., & Nasri, H. (2019). Synthesis, structure, photophysical properties and biological activity of a cobalt(II) coordination complex with 4,4′-bipyridine and porphyrin chelating ligands. Journal of Saudi Chemical Society, 23(7), 781–794. https://doi.org/10.1016/j.jscs.2019.03.003
Xu, Z. (2013). Mechanics of metal-catecholate complexes: The roles of coordination state and metal types. Scientific Reports, 3(1), 2914. https://doi.org/10.1038/srep02914
Haas, K. L., & Franz, K. J. (2009). Application of metal coordination chemistry to explore and manipulate cell biology. Chemical Reviews, 109(10), 4921–4960. https://doi.org/10.1021/cr900134a
Alghamdi, N. J., Balaraman, L., Emhoff, K. A., Salem, A. M. H., Wei, R., Zhou, A., & Boyd, W. C. (2019). Cobalt(II) diphenylazodioxide complexes induce apoptosis in SK-HEP-1 cells. ACS Omega, 4(11), 14503–14510. https://doi.org/10.1021/acsomega.9b01684
Berradj, O., Bougherra, H., Adkhis, A., Amrouche, T., Amraoui, N. E., & Hammoutène, D. (2021). Synthesis, spectroscopic, thermal decomposition, DFT studies and antibacterial activity of uracil cobalt(III)dimethylglyoximato complexes. Journal of Molecular Structure, 1232, 130040. https://doi.org/10.1016/j.molstruc.2021.130040
Lehleh, A., Beghidja, A., Beghidja, C., Welter, R., & Kurmoo, M. (2015). Synthesis, structures and magnetic properties of dimeric copper and trimeric cobalt complexes supported by bridging cinnamate and chelating phenanthroline. Comptes Rendus Chimie, 18(5), 530–539. https://doi.org/10.1016/j.crci.2014.10.002
Kucková, L., Jomová, K., Švorcová, A., Valko, M., Segľa, P., Moncoľ, J., & Kožíšek, J. (2015). Synthesis, crystal structure, spectroscopic properties and potential biological activities of salicylate-neocuproine ternary copper(II) complexes. Molecules, 20(2), 2115–2137. https://doi.org/10.3390/molecules20022115
Tong, Y.-P., & Lin, Y.-W. (2009). Synthesis, crystal structure, electronic structure, bonding, photoluminescence and spectroscopic property investigations of a mononuclear 1,10-phenanthroline and 5-bromo-salicylate ternary indium(III) complex. Inorganica Chimica Acta, 362(13), 4791–4796. https://doi.org/10.1016/j.ica.2009.07.005
Lewis, R. A., MacLeod, K. C., Mercado, B. Q., & Holland, P. L. (2014). Geometric and redox flexibility of pyridine as a redox-active ligand that can reversibly accept one or two electrons. Chemical Communications, 50(76), 11114–11117. https://doi.org/10.1039/C4CC05495D
Jesse, K. A., Filatov, A. S., Xie, J., & Anderson, J. S. (2019). Neocuproine as a redox-active ligand platform on iron and cobalt. Inorganic Chemistry, 58(14), 9057–9066. https://doi.org/10.1021/acs.inorgchem.9b00531
Muhammad, N., & Guo, Z. (2014). Metal-based anticancer chemotherapeutic agents. Current Opinion in Chemical Biology, 19(1), 144–153. https://doi.org/10.1016/J.CBPA.2014.02.003
van Niekerk, A., Chellan, P., & Mapolie, S. F. (2019). Heterometallic multinuclear complexes as anti-cancer agents-an overview of recent developments. European Journal of Inorganic Chemistry, 2019(30), 3432–3455. https://doi.org/10.1002/EJIC.201900375
De Souza, I. C. A., De Souza Santana, S., Gómez, J. G., Guedes, G. P., Madureira, J., De Ornelas Quintal, S. M., & Lanznaster, M. (2020). Investigation of cobalt(III)–phenylalanine complexes for hypoxia-activated drug delivery. Dalton Transactions, 49(45), 16425–16439. https://doi.org/10.1039/D0DT01389G
Jungwirth, U., Kowol, C. R., Keppler, B. K., Hartinger, C. G., Berger, W., & Heffeter, P. (2011). Anticancer activity of metal complexes: Involvement of redox processes. Antioxidants & redox signaling, 15(4), 1085–1127. https://doi.org/10.1089/ARS.2010.3663
Vlasiou, M. C., Ioannou, K., Eleftheriou, C., Pafiti, K. S., Zacharia, L. C., & El-Shazly, M. (2022). Synthesis and biological evaluation of a new chalconate Co (II/III) complex with cytotoxic activity. Journal of Molecular Structure, 1249, 131567. https://doi.org/10.1016/J.MOLSTRUC.2021.131567
Kou, Y.-Y., Li, M.-L., & Ren, X.-H. (2018). Synthesis, structure, and DNA binding/cleavage of two novel binuclear Co(II) complexes. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 205, 435–441. https://doi.org/10.1016/j.saa.2018.07.050
Shankar, K., & Baruah, J. B. (2017). A stable peroxo- and hydroxido-bridged dinuclear cobalt(III) ethylenediammine 2,4-dinitrophenolate complex. Inorganic Chemistry Communications, 84, 45–48. https://doi.org/10.1016/j.inoche.2017.07.006
Abebe, A., Bayeh, Y., Belay, M., Gebretsadik, T., Thomas, M., & Linert, W. (2020). Mono and binuclear cobalt(II) mixed ligand complexes containing 1,10-phenanthroline and adenine using 1,3-diaminopropane as a spacer: Synthesis, characterization, and antibacterial activity investigations. Future Journal of Pharmaceutical Sciences, 6(1), 13. https://doi.org/10.1186/s43094-020-00030-4
Abu Shamma, A., Abu Ali, H., & Kamel, S. (2018). Synthesis, characterization and biological properties of mixed ligand complexes of cobalt(II/III) valproate with 2,9-dimethyl-1,10-phenanthroline and 1,10-phenanthroline. Applied Organometallic Chemistry, 32(1), e3904. https://doi.org/10.1002/aoc.3904
Ayad, M. I. (2016). Synthesis, characterization and catechol oxidase biomimetic catalytic activity of cobalt(II) and copper(II) complexes containing N2O2 donor sets of imine ligands. Arabian Journal of Chemistry, 9, S1297–S1306. https://doi.org/10.1016/J.ARABJC.2012.02.007
Nesterov, D., & Nesterova, O. (2018). Polynuclear cobalt complexes as catalysts for light-driven water oxidation: A review of recent advances. Catalysts, 8(12), 602. https://doi.org/10.3390/catal8120602
Ahmad, N., Chughtai, A. H., Younus, H. A., & Verpoort, F. (2014). Discrete metal-carboxylate self-assembled cages: Design, synthesis and applications. Coordination Chemistry Reviews, 280, 1–27. https://doi.org/10.1016/j.ccr.2014.07.005
Hachey, A. C., Havrylyuk, D., & Glazer, E. C. (2021). Biological activities of polypyridyl-type ligands: Implications for bioinorganic chemistry and light-activated metal complexes. Current Opinion in Chemical Biology, 61, 191–202. https://doi.org/10.1016/j.cbpa.2021.01.016
Karumban, K. S., Raut, R., Gupta, P., Muley, A., Giri, B., Kumbhakar, S., & Maji, S. (2022). Mononuclear cobalt(II) complexes with polypyridyl ligands: Synthesis, characterization, DNA interactions and in vitro cytotoxicity towards human cancer cells. Journal of Inorganic Biochemistry, 233, 111866. https://doi.org/10.1016/j.jinorgbio.2022.111866
Wang, Y. F., Tang, J. X., Mo, Z. Y., Li, J., Liang, F. P., & Zou, H. H. (2022). The strong in vitro and vivo cytotoxicity of three new cobalt(ii) complexes with 8-methoxyquinoline. Dalton Transactions, 51, 8840–8847. https://doi.org/10.1039/d2dt01310j
Liu, S. J., Xue, L., Hu, T. L., & Bu, X. H. (2012). Two new CoII coordination polymers based on carboxylate-bridged di- and trinuclear clusters with a pyridinedicarboxylate ligand: Synthesis, structures and magnetism. Dalton Transactions, 41(22), 6813–6819. https://doi.org/10.1039/C2DT30297G
Ghosh, I., Chakraborty, B., Bera, A., Paul, S., & Paine, T. K. (2022). Selective oxygenation of C-H and CC bonds with H2O2 by high-spin cobalt(II)-carboxylate complexes. Dalton Transactions, 51(6), 2480–2492. https://doi.org/10.1039/D1DT02235K
Kamaal, S., Ali, A., Afzal, M., Muslim, M., Alarifi, A., & Ahmad, M. (2022). Exploiting the biological potential of Zn(II) complex derived from zwitterionic Schiff base: DNA binding and cytotoxicity activity against human cervical cancer. Chemical Papers. https://doi.org/10.1007/S11696-022-02243-8
Ali, A., Mishra, S., Kamaal, S., Alarifi, A., Afzal, M., Saha, K. D., & Ahmad, M. (2021). Evaluation of catacholase mimicking activity and apoptosis in human colorectal carcinoma cell line by activating mitochondrial pathway of copper(II) complex coupled with 2-(quinolin-8-yloxy)(methyl)benzonitrile and 8-hydroxyquinoline. Bioorganic Chemistry, 106, 104479. https://doi.org/10.1016/J.BIOORG.2020.104479
Ali, A., Banerjee, S., Kamaal, S., Usman, M., Das, N., Afzal, M., & Ahmad, M. (2021). Ligand substituent effect on the cytotoxicity activity of two new copper( ii ) complexes bearing 8-hydroxyquinoline derivatives: Validated by MTT assay and apoptosis in MCF-7 cancer cell line (human breast cancer). RSC Advances, 11(24), 14362–14373. https://doi.org/10.1039/D1RA00172H
Sepay, N., Saha, P. C., Shahzadi, Z., Chakraborty, A., & Halder, U. C. (2021). A crystallography-based investigation of weak interactions for drug design against COVID-19. Physical Chemistry Chemical Physics, 23(12), 7261–7270. https://doi.org/10.1039/D0CP05714B
MacGillavry, C. (1962). International tables for X-ray crystallography. Volume III, Physical and chemical tables. Birmingham: Kynoch press.
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., & Puschmann, H. (2009). OLEX2: A complete structure solution, refinement and analysis program. Journal of Applied Crystallography, 42(2), 339–341. https://doi.org/10.1107/S0021889808042726
Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K., & Puschmann, H. (2015). The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment – Olex2 dissected. Acta Crystallographica Section A, 71(1), 59–75. https://doi.org/10.1107/S2053273314022207
Blatov, V. A., Shevchenko, A. P., & Proserpio, D. M. (2014). Applied topological analysis of crystal structures with the program package topospro. Crystal Growth and Design, 14(7), 3576–3586. https://doi.org/10.1021/CG500498K/ASSET/IMAGES/LARGE/CG-2014-00498K_0017.JPEG
Alexandrov, E. V., Blatov, V. A., Kochetkov, A. V., & Proserpio, D. M. (2011). Underlying nets in three-periodic coordination polymers: Topology, taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database. CrystEngComm, 13(12), 3947–3958. https://doi.org/10.1039/c0ce00636j
Blatov, V. A. (2016). A method for topological analysis of rod packings. Structural Chemistry, 27(6), 1605–1611. https://doi.org/10.1007/S11224-016-0774-1
Sousa, S. F., Fernandes, P. A., & Ramos, M. J. (2006). Protein-ligand docking: Current status and future challenges. Proteins: Structure, Function, and Bioinformatics, 65(1), 15–26. https://doi.org/10.1002/prot.21082
Waterhouse, A., Bertoni, M., Bienert, S., Studer, G., Tauriello, G., Gumienny, R., & Schwede, T. (2018). SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic acids research, 46(W1), W296–W303. https://doi.org/10.1093/nar/gky427
Phillips, J. C., Hardy, D. J., Maia, J. D. C., Stone, J. E., Ribeiro, J. V., Bernardi, R. C., & Tajkhorshid, E. (2020). Scalable molecular dynamics on CPU and GPU architectures with NAMD. The Journal of Chemical Physics, 153(4), 044130. https://doi.org/10.1063/5.0014475
Muslim, M., Ali, A., Neogi, I., Dege, N., Shahid, M., & Ahmad, M. (2021). Facile synthesis, topological study, and adsorption properties of a novel Co (II)-based coordination polymer for adsorptive removal of methylene blue and methyl orange dyes. Polyhedron, 210, 115519. https://doi.org/10.1016/J.POLY.2021.115519
Muslim, M., Ali, A., Ahmad, M., Alarifi, A., Afzal, M., Sepay, N., & Dege, N. (2022). A zinc(II) metal–organic complex based on 2-(2-aminophenyl)-1H-benzimidazole ligand: Exhibiting high adsorption capacity for aromatic hazardous dyes and catecholase mimicking activity. Journal of Molecular Liquids, 363, 119767. https://doi.org/10.1016/J.MOLLIQ.2022.119767
Muslim, M., Faizi, S. H., Ali, A., Afzal, M., Ahmad, M., Dege, N., & Mashrai, A. (2021). Crystal structure and Hirshfeld surface analysis of [2-(1H-benzimidazol-2-yl-κ N 3)aniline-κ N]dichloridozinc(II) N, N-dimethylformamide monosolvate. Acta Crystallographica Section E: Crystallographic Communications, 77(Ii), 491–494. https://doi.org/10.1107/S2056989021003649
M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, P. R. Spackman, D. Jayatilaka, & M. A. Spackman, (2017). CrystalExplorer17. University of Western Australia. http://hirshfeldsurface.Net
Suresh, M., & Srinivasan, K. (2022). Crystal structure and Hirshfeld surface analysis of DL-methionine polymorphs (α and β). Journal of Molecular Structure, 1250(1), 131721. https://doi.org/10.1016/J.MOLSTRUC.2021.131721
Muslim, M., Kamaal, S., Ahmad, M., Arish, M., Jane Alam, M., Kumar Pradhan, A., & Afzal, M. (2022). Structural elucidation and cytotoxicity profile of neocuproine-Cu(II) and Cu(I)-based chemotherapeutic agents: Effect of picric acid-derived cocrystals. Polyhedron, 220, 115848. https://doi.org/10.1016/J.POLY.2022.115848
Muslim, M., Ahmad, M., Arish, M., Alam, M. J., Alarifi, A., Afzal, M., & Ahmad, S. (2022). 5-Hydroxyisophthalic acid and neocuproine containing copper(II) complex as a promising cytotoxic agent: Structure elucidation, topology, Hirshfeld surface, DFT calculations, and molecular docking analysis. Journal of Molecular Structure, 1270, 133879. https://doi.org/10.1016/J.MOLSTRUC.2022.133879
Pap, J. S., Kripli, B., Giorgi, M., Kaizer, J., & Speier, G. (2011). Redox properties of cobalt(II) complexes with isoindoline-based ligands. Transition Metal Chemistry, 36(5), 481–487. https://doi.org/10.1007/S11243-011-9493-Z
Acknowledgements
The Department of Applied Chemistry, Faculty of Engineering and Technology, Aligarh Muslim University, UP, India, and Researchers Supporting Project number (RSP-2021/288), King Saud University, Riyadh, Saudi Arabia, are gratefully acknowledged for financial support and laboratory facilities. UGC Start-Up grant and TEQIP-III, ZHCET, Aligarh Muslim University, is thanked for the extensive help in procuring chemicals.
Funding
A Start-Up Grant from the UGC, India, and Researchers Supporting Project number (RSP-2021/288), King Saud University, Riyadh, Saudi Arabia, is gratefully acknowledged for financial support.
Author information
Authors and Affiliations
Contributions
All authors authorize that they have contributed reasonably, and take full liability for the work. Mohd Muslim (M. Muslim): writing—original draft; formal analysis; validation; writing—review and editing; project administration. Farha Naz (F. Naz): data curation, formal analysis. Abdullah Alarifi (A. Alarifi): formal analysis. Mohd. Afzal (M. Afzal): formal analysis. Nayim Sepay (N. Sepay): formal analysis, software. Musheer Ahmad (M. Ahmad): supervision, data curation, project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
The authors declare that they consent to participate.
Consent to Publish
The authors declare that they consent to publication.
Declarations of Author’s Agreement to Authorship and Submission
All authors have agreed to authorship and the submission of this manuscript for peer review and publication in the Applied Biochemistry and Biotechnology Journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muslim, M., Naz, F., Alarifi, A. et al. Structural, Theoretical Investigations, Hirshfeld Surface Analysis, and Cytotoxicity Profile of a Neocuproine-Co(II)-Based Discrete Homodinuclear Complex. Appl Biochem Biotechnol 195, 871–888 (2023). https://doi.org/10.1007/s12010-022-04180-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04180-4