Abstract
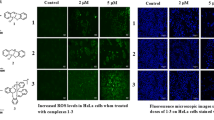
A new trinuclear Cu(II) complex [Cu3(μ3-OH)(μ-pz)3(Hpz)2(HCOO)(BTAO)Cl] (1) (Hpz = pyrazole, BTAO = 4,6-bis(dimethylamino)-1,3,5-triazin-2(1H)-one) and four mononuclear complexes [M(Hpz)4Cl2] [M2+ = Cu2+, Co2+, Ni2+ and Zn2+] (2–5) have been synthesized and characterized. Electronic absorption spectra, fluorescence spectra and circular dichroism spectra were carried out to investigate the interactions between complexes 1–5 and CT-DNA. These complexes exhibited different binding affinity with DNA, and the order of DNA binding strength was 1 > 2 > 4 > 3 > 5. Most of these complexes could cleave pBR322 DNA efficiently in the presence of H2O2 as an activator, and the cleavage efficacy of these complexes was quite consistent with their DNA binding abilities. Furthermore, the in vitro cytotoxicity studies of complexes 1–5 were measured by MTT assay. The trinuclear Cu(II) complex 1 showed cytotoxicity against human lung carcinoma cell lines A549 (IC50 = 24.314 ± 1.386 µM) and human esophageal carcinoma cell line EC109 (IC50 = 27.335 ± 1.437 µM), while the mononuclear complexes 2–5 did not show apparent cytotoxicity to the tested cell lines (IC50 > 50 μM). The different cytotoxicity of these complexes may also be relevant to their reactivity toward DNA.









Similar content being viewed by others
References
Ndagi U, Mhlongo N, Soliman ME (2017) Drug Des Dev Ther 11:599–616
Liang JX, Zhong HJ, Yang G, Vellaisamy K, Ma DL, Leung CH (2017) J Inorg Biochem 177:276–286
Muhammad N, Guo ZJ (2014) Curr Opin Chem Biol 19:144–153
Cai L, Yu C, Ba L, Liu Q, Qian Y, Yang B, Gao C (2018) Appl Organomet Chem 32(4): e4228.
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Chem Rev 114(1):815–862
Porchia M, Pellei M, Del Bello F, Santini C (2020) Molecules 25(24):5814
Barone G, Terenzi A, Lauria A, Almerico AM, Leal JM, Busto N, García B (2013) Coordin Chem Rev 257:2848–2862
Wang HL, Sorolla M, Wang XQ, Jacobson AJ, Wang HY, Pillai AK (2019) Transit Metal Chem 44(3):237–245
Parveen S, Arjmand F, Tabassum S (2019) Eur J Med Chem 175:269–286
Li D, Wu P, Sun N, Lu YJ, Wong WL, Fang Z, Zhang K (2019) Curr Org Chem 23(5):616–627
Bennani FE, Doudach L, Cherrah Y, Ramli Y, Karrouchi K, Ansar M, Faouzi ME (2020) Bioorg Chem 97:103470
Khan MF, Alam MM, Verma G, Akhtar W, Akhter M, Shaquiquzzaman M (2016) Eur J Med Chem 120:170–201
Sahoo J, Sahoo CR, Nandini Sarangi PK, Prusty SK, Padhy RN, Paidesetty SK (2020) Eur J Med Chem 186:111911
Keter FK, Darkwa J (2012) Biometals 25(1):9–21
Azam M, Wabaidur SM, Alam M, Khan Z, Alanazi IO, Al-Resayes SI, Moon IS (2020) Transit Metal Chem 46(1):65–71
Matada MN, Jathi K (2019) J Coord Chem 72(12):1994–2014
Pellei M, Bagnarelli L, Luciani L, Del Bello F, Giorgioni G, Piergentili A, Quaglia W, De Franco M, Gandin V, Marzano C, Santini C (2020) Int J Mol Sci 21(7):2616
Jana A, Brandao P, Mondal G, Bera P, Santra A (2018) Inorg Chim Acta 482:621–634
Zhou HP, Gan XP, Li XL, Liu ZD, Geng WQ, Zhou FX, Ke WZ, Wang P, Kong L, Hao FY, Wu JY, Tian YP (2010) Crystal Growth Des 10(4):1767–1776
Kazemi Z, Amiri Rudbari H, Mirkhani V, Sahihi M, Moghadam M, Tangestaninejad S, Mohammadpoor Baltork I, Kajani AA, Azimi G (2017) Eur J Med Chem 135:230–240
Zhao J, Li S, Wang X, Xu G, Gou S (2019) Inorg chem 58(3):2208–2217
Fang ZW, Yan J, Yu WD, Zhang N, Zhang SC (2019) Transit Metal Chem 44(5):463–474
Zheng L, Qiu X, Xu Y, Fu J, Yuan Y, Zhu D, Chen S (2011) CrystEngComm 13(7):2714–2720
Burrows AD, Cassar K, Mahon MF, Rigby SP, Warren JE (2005) CrystEngComm 7:548–550
Manzur J, Acuna C, Vega A (2011) Inorg Chim Acta 374(1):637–642
Wang JX, Wang C, Wang X, Wang XY, Xing YH (2015) Spectrochim Acta Part A 142:55–61
Daugherty NA, Swisher JH (1968) Inorg Chem 7(8):1651–1653
Casarin M, Corvaja C, Di Nicola C, Falcomer D, Franco L, Monari M, Pandolfo L, Pettinari C, Piccinelli F, Tagliatesta P (2004) Inorg Chem 43(19):5865–5876
Zhang Q, Zhang Q, Li ZZ, Liu H, Liu JC (2020) Dyes and Pigments 173: 107923.
Han G, Yang P (2002) J Inorg Biochem 91(1):230–236
Protas AV, Popova EA, Mikolaichuk OV, Porozov YB, Mehtiev AR, Ott I, Alekseev GV, Kasyanenko NA, Trifonov RE (2018) Inorg Chim Acta 473:133–144
Zhu LN, Gao HR, Wang HX, Xu MY, Li XZ (2014) Eur J Inorg Chem 2014(14):2396–2405
Tjioe L, Meininger A, Joshi T, Spiccia L, Graham B (2011) Inorg Chem 50(10):4327–4339
Giffard D, Fischer Fodor E, Vlad C, Achimas Cadariu P, Smith GS (2018) Eur J Med Chem 157:773–781
Anbu S, Kandaswamy M (2011) Polyhedron 30(1):123–131
Acknowledgments
This work was supported by the Natural Science Foundation of Hunan Province (Grant 2020JJ4684).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, X., Nie, Y., Tang, Q. et al. Pyrazole-based trinuclear and mononuclear complexes: synthesis, characterization, DNA interactions and cytotoxicity studies. Transit Met Chem 46, 481–494 (2021). https://doi.org/10.1007/s11243-021-00466-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-021-00466-4