Abstract
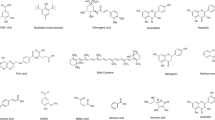
The present study was focused on the screening of phytochemicals, their quantitative estimation and analysis by LC–MS profile, and antiproliferative efficacy of the aqueous-ethanolic extracts of the microalgae, Chlorochromonas danica isolated from the freshwater body Tavanampalli. The aqueous-ethanol extract of Chlorochromonas danica showed the presence of flavonoids, phenols, and proteins. The total flavonoid content, total phenol content, and total protein content were determined to be 158.65 mg of quercetin equivalent, 15.75 mg of gallic acid equivalent, and 134.65 mg/g dry weight of the extract, respectively. The LC–MS analysis confirmed the presence of several major bioactive molecules including l-Histidine, d-glutamine, l-aspartic acid, adenine, adenosine, cotinine, guanine hypoxanthine, l-glutamic acid, nicotinamide, 4-Hydroxycoumarin, and Stearamide. The aqueous-ethanol extract of Chlorochromonas danica exhibited an IC50 values of 63.34 µg, 279.29 µg, 125.42 µg, 90.56 µg, and 95.58 µg against A375, A549, HeLa, HepG2, and HT29 cell lines respectively, compared to the positive control cisplatin with IC50 values of 3.56 µg, 4.65 µg, 3.88 µg, 4.87 µg, and 7.23 µg respectively. These data suggest that Chlorochromonas danica remains a promising drug candidate for the treatment of cancers, particularly melanoma (A375 cell line) that can be considered for purification of antiproliferative compound and further clinical trials for the discovery of novel antiproliferative drugs from cost-effective sources.












Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barsanti, L., Coltelli, P., Evangelista, V., Frassanito, A. M., Passarelli, V., Vesentini, N., & Gualtieri, P. (2008). Oddities and curiosities in the algal world. In: ALgal Toxins: Nature, Occurence Effect and Deltection NATO Science for Peace and Security Series A: Chemistry and Biology Springer, Dordrecht. https://doi.org/10.1201/b16544-11
Scieszka, S., & Klewicka, E. (2018). Algae in food: a general review. Critical Reviews in Food Science and Nutrition, 59(21), 3538–3547. https://doi.org/10.1080/10408398.2018.1496319
Chakdar, H., & Pabbi, S. (2017). Algal pigments for human health and cosmeceutivals. Algal Green Chemistry. pp. 171–188. https://doi.org/10.1016/B978-0-444-63784-0.00009-6
Tang, D. Y. Y., Khoo, K. S., Chew, K. W., Tao, Y., Ho, S. H., & Show, P. L. (2020). Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresource Technology, 304, 122997. https://doi.org/10.1016/j.biortech.2020.122997
Koyande, A. K., Chew, K. W., Rambabu, K., Tao, Y., Chu, D. T., & Show, P. L. (2019). Microalgae: A potential alternative to health supplementation for humans. Food Science and Human Wellness, 8(1), 16–24. https://doi.org/10.1016/j.fshw.2019.03.001
Khan, M. I., Shin, J. H., & Kim, J. D. (2018). The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Factories, 17(1), 1–21. https://doi.org/10.1186/s12934-018-0879-x
Semple, K. T., & Cain, R. B. (1996). Biodegradation of phenols by the alga Ochromonas danica. Applied and Environmental Microbiology, 62(4), 1265–1273. https://doi.org/10.1128/aem.62.4.1265-1273.1996
Mitra, A., Flynn, K. J., Tillmann, U., Raven, J. A., Caron, D., Stoecker, D. K., … Lundgren, V. (2016). Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: Incorporation of diverse mixotrophic strategies. Protist 167(2):106–120. https://doi.org/10.1016/j.protis.2016.01.003
Pulz, O., & Gross, W. (2004). Valuable products from biotechnology of microalgae. Applied Microbiology and Biotechnology, 65(6), 635–648. https://doi.org/10.1007/s00253-004-1647-x
Sathasivam, R., Radhakrishnan, R., Hashem, A., & Abd-Allah, E. F. (2019). Microalgae metabolites: A rich source for food and medicine. Saudi Journal of Biological Sciences, 26(4), 709–722. https://doi.org/10.1016/j.sjbs.2017.11.003
Bogen, C., Klassen, V., Wichmann, J., Russa, M. La, Doebbe, A., Grundmann, M., … Mussgnug, J. H. (2013). Identification of monoraphidium contortum as a promising species for liquid biofuel production. Bioresource Technology 133:622–626. https://doi.org/10.1016/j.biortech.2013.01.164
Invally, K., & Ju, L. K. (2017). Biolytic effect of rhamnolipid biosurfactant and dodecyl sulfate against phagotrophic alga Ochromonas danica. Journal of Surfactants and Detergents, 20(5), 1161–1171. https://doi.org/10.1007/s11743-017-2005-1
Nicholls, K. H., & Wujek, D. E. (2003). Chrysophycean Algae. In: Freshwater Algae of North America: Ecology and Classification. pp. 471–509. https://doi.org/10.1016/B978-012741550-5/50013-1
Lin, Z., Li, C., & Ju, L. K. (2019). Glycerol and acetate additions to maximize lipid content in high-density cultures of phagotrophic alga ochromonas danica. Journal of the American Oil Chemists’ Society, 96(3), 231–238. https://doi.org/10.1002/aocs.12183
Lie, A. A. Y., Liu, Z., Terrado, R., Tatters, A. O., Heidelberg, K. B., & Caron, D. A. (2017). Effect of light and prey availability on gene expression of the mixotrophic chrysophyte Ochromonas sp. BMC Genomics, 18(1), 1–16. https://doi.org/10.1186/s12864-017-3549-1
Demirtas, I., Erenler, R., Elmastas, M., & Goktasoglu, A. (2013). Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chemistry, 136(1), 34–40. https://doi.org/10.1016/j.foodchem.2012.07.086
Yaglıoglu, A. S., Akdulum, B., Erenler, R., Demirtas, I., Telci, I., & Tekin, S. (2013). Antiproliferative activity of pentadeca-(8E, 13Z) dien-11-yn-2-one and (E)-1,8-pentadecadiene from Echinacea pallida (Nutt.) Nutt. roots. Medicinal Chemistry Research, 22(6), 2946–2953. https://doi.org/10.1007/s00044-012-0297-2
Erenler, R., Sen, O., SahinYaglioglu, A., & Demirtas, I. (2016). Bioactivity-guided isolation of antiproliferative sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Combinatorial Chemistry & High Throughput Screening, 19(1), 66–72. https://doi.org/10.2174/1386207319666151203002117
Forni, C., Facchiano, F., Bartoli, M., Pieretti, S., Facchiano, A., D’Arcangelo, D., … Jadeja, R. N. (2019). Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Research International, 2019(Figure 1). https://doi.org/10.1155/2019/8748253
Lee, J., Baek, S., Lee, J., Lee, J., Lee, D. G., Park, M. K., … Kwok, S. K. (2015). Digoxin ameliorates autoimmune arthritis via suppression of Th17 differentiation. International Immunopharmacology 26(1):103–111. https://doi.org/10.1016/j.intimp.2015.03.017
Meletis, C. D., & Barker, J. E. (2005). Therapeutic uses of amino acids. Alternative and Complementary Therapies, 11(1), 24–28. https://doi.org/10.1089/act.2005.11.24
Wang, W., & Zou, W. (2020). Amino acids and their transporters in T cell immunity and cancer therapy. Molecular Cell, 80(3), 384–395. https://doi.org/10.1016/j.molcel.2020.09.006
El-Hack, M. E., Abdelnour, S., Alagawany, M., Abdo, M., Sakr, M. A., Khafaga, A. F., … Gebriel, M. G. (2019). Microalgae in modern cancer therapy: Current knowledge. Biomedicine & Pharmacotherapy 111:42–50. https://doi.org/10.1016/j.biopha.2018.12.069
Dahiya, S., Shilpie, A., Balasundaram, G., Chowdhury, R., Kumar, P., & Mishra, A. K. (2021). Diversity of algal species present in waste stabilisation ponds and different factors affecting its enrichment and phototaxis. Chemistry and Ecology, 37(6), 515–529. https://doi.org/10.1080/02757540.2021.1910242
Dineshkumar, R., Narendran, R., Jayasingam, P., & Sampathkumar, P. (2017). Cultivation and chemical composition of microalgae Chlorella vulgaris and its antibacterial activity against human pathogens. Journal of Aquaculture & Marine Biology, 5(3), 0019. https://doi.org/10.15406/jamb.2017.05.00119
Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., & Ju, Y. H. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Journal of Food and Drug Analysis, 22(3), 296–302. https://doi.org/10.1016/j.jfda.2013.11.001
Harborne, J. B. (1973). Phytochemical Methods-a guide to modern techniques of plant analysis. pp. 33–80. https://doi.org/10.2307/4108146
Ali, A. M. A., El-Nour, M. E. A. M., & Yagi, S. M. (2018). Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. Journal of Genetic Engineering and Biotechnology, 16(2), 677–682. https://doi.org/10.1016/j.jgeb.2018.03.003
Chandra, S., Kham, S., Avula, B., Lata, H., Yang, M. A., & Khan, I. A. (2014). Assessment of total phenolic and flavanoid content, antioxidant properties and yield of aeroponically and conventionall grown leafy vegetable and fruits crops: A comparitive study. Evidence-based Complementary and Alternative Medicine 253875. https://doi.org/10.1155/2014/253875
Sarkar, S., Mondal, M., Ghosh, P., Saha, M., & Chatterjee, S. (2020). Quantification of total protein content from some traditionally used edible plant leaves: A comparative study. Journal of Medicinal Plants Studies, 8(4), 166–170. https://doi.org/10.22271/plants.2020.v8.i4c.1164
Kumar, S., Singh, A., & Kumar, B. (2017). Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. Journal of Pharmaceutical Analysis, 7(4), 214–222. https://doi.org/10.1016/j.jpha.2017.01.005
Sodde, V. K., Lobo, R., Kumar, N., Maheshwari, R., & Shreedhara, C. S. (2015). Cytotoxic activity of Macrosolen parasiticus (L.) Danser on the growth of breast cancer cell line (MCF-7). Pharmacognosy Magazine, 11(42), S156–S160. https://doi.org/10.4103/0973-1296.157719
Tang, H., Zhu, S. S., Wang, N., Xu, Z., Huang, J., Gu, L., … Huang, Y. (2020). The inhibitory effect of mixotrophic Ochromonas gloeopara on the survival and reproduction of Daphnia similoides sinensis. Environmental Science and Pollution Research 27(23):29068–29074. https://doi.org/10.1007/s11356-020-09291-1
Boenigk, J., Pfandl, K., Stadler, P., & Chatzinotas, A. (2005). High diversity of the “Spumella-like” flagellates: An investigation based on the SSU rRNA gene sequences of isolates from habitats located in six different geographic regions. Environmental Microbiology, 7(5), 685–697. https://doi.org/10.1111/j.1462-2920.2005.00743.x
Andersen, R. A., Graf, L., Malakhov, Y., & Yoon, H. S. (2017). Rediscovery of the ochromonas type species ochromonas triangulata (chrysophyceae) from its type locality (Lake Veysove, Donetsk region, Ukraine). Phycologia, 56(6), 591–604. https://doi.org/10.2216/17-15.1
Elloumi, J., Carrias, J. F., Ayadi, H., Sime-Ngando, T., & Bouaïn, A. (2009). Communities structure of the planktonic halophiles in the solar saltern of Sfax, Tunisia. Estuarine, Coastal and Shelf Science, 81(1), 19–26. https://doi.org/10.1016/j.ecss.2008.09.019
Segal, R. D., Waite, A. M., & Hamilton, D. P. (2006). Transition from planktonic to benthic algal dominance along a salinity gradient. Hydrobiologia, 556(1), 119–135. https://doi.org/10.1007/s10750-005-0916-8
Zaremba, L. S., & Smolenski, W. H. (2000). Optimal portfolio choice under a liability constraint. Annals of Operations Research 97(1–4), 131–141. 10.1023/A.
Schmidtke, A., Bell, E. M., & Weithoff, G. (2006). Potential grazing impact of the mixotrophic flagellate Ochromonas sp. (Chrysophyceae) on bacteria in an extremely acidic lake. Journal of Plankton Research, 28(11), 991–1001. https://doi.org/10.1093/plankt/fbl034
Dai, J., & Mumper, R. J. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules, 15(10), 7313–7352. https://doi.org/10.3390/molecules15107313
Turkmen, N., Sari, F., & Velioglu, Y. S. (2006). Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chemistry, 99(4), 835–841. https://doi.org/10.1016/j.foodchem.2005.08.034
Cagalj, M., Skroza, D., Tabanelli, G., Özogul, F., & Šimat, V. (2021). Maximizing the antioxidant capacity of Padina pavonica by choosing the right drying and extraction methods. Processes, 9(4), 1–15. https://doi.org/10.3390/pr9040587
Widowati, I., Zainuri, M., Kusumaningrum, H. P., Susilowati, R., Hardivillier, Y., Leignel, V., … Mouget, J. L. (2017). Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone Tahiti. IOP Conference Series: Earth and Environmental Science 55:1–6. https://doi.org/10.1088/1755-1315/55/1/012067
Stanchev, S., Jensen, F., Hinkov, A., Atanasov, V., Genova-Kalou, P., Argirova, R., & Manolov, I. (2011). Synthesis and Inhibiting Activity of Some 4 Hydroxycoumarin Derivatives on HIV-I Protease. ISRN Pharmaceutics. https://doi.org/10.5402/2011/137637
Saleem, A., Bukhari, S. M., Zaidi, A., Farooq, U., Ali, M., Khan, A., … Khan, F. A. (2020). Enzyme inhibition and antibacterial potential of 4-hydroxycoumarin derivatives. Brazilian Journal of Pharmaceutical Sciences 56:1–10. https://doi.org/10.1590/s2175-97902019000418654
Banday, S. M., Showkat, M., & Khan, K. Z. (2019). Structure-activity relationship of anticancer potential of 4-hydroxycoumarin and its derivatives: A comparative study. Asian Journal of Pharmacy and Pharmacology, 5(3), 470–479. https://doi.org/10.31024/ajpp.2019.5.3.7
Zavrsnik, D., Muratovic, S., Spirtovic, S., Softic, D., & Medic-Saric, M. (2008). The synthesis and antimicrobial activity of some 4-hydroxycoumarin derivatives. Bosnian Journal of Basic Medical Sciences, 8(3), 277–281. https://doi.org/10.17305/bjbms.2008.2933
Akkol, E. K., Genç, Y., Karpuz, B., Sobarzo-Sánchez, E., & Capasso, R. (2020). Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers, 12(7), 1–25. https://doi.org/10.3390/cancers12071959
Wang, C., Song, Z., Yu, H., Liu, K., & Ma, X. (2015). Adenine: An important drug scaffold for the design of antiviral agents. Acta Pharmaceutica Sinica B, 5(5), 431–441. https://doi.org/10.1016/j.apsb.2015.07.002
Peck, C. C., Moore, G. L., Bolin, R. B., & Dawson, R. B. (1981). Adenine blood preservation. Critical Reviews in Clinical Laboratory Sciences, 13(3), 173–212. https://doi.org/10.3109/10408368109106447
Nishino, T., Yachie-Kinoshita, A., Hirayama, A., Soga, T., Suematsu, M., & Tomita, M. (2013). Dynamic simulation and metabolome analysis of long-term erythrocyte storage in adenine-guanosine solution. PLoS ONE, 8(8), e71060. https://doi.org/10.1371/journal.pone.0071060
Sadigh-Eteghad, S., Vatandoust, S. M., Mahmoudi, J., Rahigh Aghsan, S., & Majdi, A. (2020). Cotinine ameliorates memory and learning impairment in senescent mice. Brain Research Bulletin, 164(June), 65–74. https://doi.org/10.1016/j.brainresbull.2020.08.010
Grizzell, J. A., Patel, S., Barreto, G. E., & Echeverria, V. (2017). Cotinine improves visual recognition memory and decreases cortical Tau phosphorylation in the Tg6799 mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 78, 75–81. https://doi.org/10.1016/j.pnpbp.2017.05.010
Stefan, L., & Monchaud, D. (2019). Applications of guanine quartets in nanotechnology and chemical biology. Nature Reviews Chemistry, 3(11), 650–668. https://doi.org/10.1038/s41570-019-0132-0
Kaucher, M. S., Harrell, W. A., & Davis, J. T. (2006). A unimolecular G-quadruplex that functions as a synthetic transmembrane Na+ transporter. Journal of the American Chemical Society, 128(1), 38–39. https://doi.org/10.1021/ja056888e
Edelman, J. J. B., Seco, M., Dunne, B., Matzelle, S. J., Murphy, M., Joshi, P., … Passage, J. (2013). Custodiol for myocardial protection and preservation: a systematic review. Annals of Cardiothoracic Surgery, 2(6):717–728. https://doi.org/10.3978/j.issn.2225-319X.2013.11.10
Holecek, M. (2020). Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement Milan. Nutrients, 12(848). https://doi.org/10.3390/nu12030848
Bampidis, V., Azimonti, G., Bastos, M. L., Christensen, H., Dusemund, B., Kouba, M., … Ramos, F. (2019). Safety and efficacy of l‐histidine monohydrochloride monohydrate produced using Corynebacterium glutamicum KCCM 80172 for all animal species. European Food Safety Authority 17(7):1–20. https://doi.org/10.2903/j.efsa.2019.5783
Desjardins-Park, H. E., Foster, D. S., & Longaker, M. T. (2018). Fibroblasts and wound healing: An update. Regenerative Medicine, 13(5), 491–495. https://doi.org/10.2217/rme-2018-0073
Takahashi, S., Saegusa, J., Sendo, S., Okano, T., Akashi, K., Irino, Y., & Morinobu, A. (2017). Glutaminase 1 plays a key role in the cell growth of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Research and Therapy, 19(1), 1–10. https://doi.org/10.1186/s13075-017-1283-3
Wang, Z., Wang, K., Feng, Y., Jiang, S., Zhao, Y., & Zeng, M. (2020). Preparation, characterization of L-aspartic acid chelated calcium from oyster shell source and its calcium supplementation effect in rats. Journal of Functional Foods, 75(5), 104249. https://doi.org/10.1016/j.jff.2020.104249
Ichikawa, S., Gohda, T., Murakoshi, M., Li, Z., Adachi, E., Koshida, T., & Suzuki, Y. (2020). Aspartic acid supplementation ameliorates symptoms of diabetic kidney disease in mice. FEBS Open Bio, 10(6), 1122–1134. https://doi.org/10.1002/2211-5463.12862
Fardus, J., Hossain, M. S., & Fujita, M. (2021). Modulation of the antioxidant defense system by exogenous l-glutamic acid application enhances salt tolerance in lentil (Lens culinaris medik.). Biomolecules, 11(4), 587. https://doi.org/10.3390/biom11040587
Chandra, K., Gopi Rao, D. V., Subramanian, K. A., & Valarmathi, K. (2018). Current status of freshwater biodiversity of India : an over view faunal diversity of India, 5, 1–23.
Sundar, S., Heino, J., Roque, F. de O., Simaika, J. P., Melo, A. S., Tonkin, J. D., … Silva, D. P. (2020). Conservation of freshwater macroinvertebrate biodiversity in tropical regions. Aquatic Conservation: Marine and Freshwater Ecosystems 30(6), 1238–1250. https://doi.org/10.1002/aqc.3326
White, A. R., Duggan, B. M., Tsai, S. C., & Vanderwal, C. D. (2016). The Alga Ochromonas danica produces Bromosulfolipids. Organic Letters, 18(5), 1124–1127. https://doi.org/10.1021/acs.orglett.6b00230.The
Moss, F. R., Cabrera, G. E., McKenna, G. M., Salerno, G. J., Shuken, S. R., Landry, M. L., … Boxer, S. G. (2020). Halogenation-dependent effects of the chlorosulfolipids of Ochromonas danica on lipid bilayers. ACS Chemical Biology 15(11), 2986–2995. https://doi.org/10.1021/acschembio.0c00624
Guo, Q., Yu, J., Zhao, Y., Liu, T., Su, M., Jia, Z., … Yang, M. (2019). Identification of fishy odor causing compounds produced by Ochromonas sp. and Cryptomonas ovate with gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. Science of the Total Environment 671:149–156. https://doi.org/10.1016/j.scitotenv.2019.03.370
Mishra, V. K., & Kumar, N. (2017). Microbial degradation of phenol—a review. Journal of Water Pollution & Purification Research, 4(1), 16–22.
Suh, S. S., Yang, E. J., Lee, S. G., Youn, U. J., Han, S. J., Kim, I. C., & Kim, S. (2017). Bioactivities of ethanol extract from the antarctic freshwater microalga, chloromonas sp. International Journal of Medical Sciences, 14(6), 560–569. https://doi.org/10.7150/ijms.18702
Parthasarathi, P., Umamaheswari, A., Banupriya, R., & Elumalai, S. (2021). Phytochemical screening and in-vitro anticancer activity of ethyl acetate fraction of Seagrass Halodule uninervis from Mandapam Coastal Region Rameswaram Gulf of Mannar India. International Journal of Pharmaceutical Sciences and Drug Research, 13(6), 677–684. https://doi.org/10.25004/IJPSDR.2021.130611
Gangadhar, K. N., Rodrigues, M. J., Pereira, H., Gaspar, H., Malcata, F. X., Barreira, L., & Varela, J. (2020). Anti-hepatocellular carcinoma (HepG2) activities of monoterpene hydroxy lactones isolated from the marine microalga tisochrysis lutea. Marine Drugs, 18(567), 1–10. https://doi.org/10.3390/md18110567
Erenler, R., Pabuccu, K., Yaglioglu, A. S., Demirtas, I., & Gul, F. (2016). Chemical constituents and antiproliferative effects of cultured Mougeotia nummuloides and Spirulina major against cancerous cell lines. Zeitschrift fur Naturforschung - Section C Journal of Biosciences, 71(3–4), 87–92. https://doi.org/10.1515/znc-2016-0010
Huang, T. H., Chiu, Y. H., Chan, Y. L., Chiu, Y. H., Wang, H., Huang, K. C., … Wu, C. J. (2015). Prophylactic administration of Fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis Tumor-bearing mice. Marine Drugs 13:1880–1900. https://doi.org/10.3390/md13041882
Tavares-Carreon, F., Torre-Zavala, S. D., Arocha-Garza, H. F., Souza, V., Galan-Wong, L. J., & Aviles-Arnaut, H. (2020). In vitro anticancer activity of methanolic extract of Granulocystopsis sp., a microalgae from an oligotrophic oasis in the Chihuahuan desert. PeerJ, 8, e8686. https://doi.org/10.7717/peerj.8686
Acknowledgements
This work was financially supported by UGC under National Fellowship for Persons with Disabilities (NFPWD)-2018-20 (ID: NFPWD-2018-20-AND-6934).The authors would like thank the Head, Department of Plant Biology and Biotechnology, Presidency College (Autonomous ), Chennai. We are grateful to Exonn Biosciences Crescent Innovation and Incubation Council, Chennai and Greensmed Labs, Chennai for their timely help throughout the research.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Geethanjali Kilari conceptualized the project’s primary principles, drafted the analysis methods, conducted the scientific investigation, formal analysis, data curation, and acquired funding from the UGC National Fellowship for Persons with Disabilities NFPWD-2018–20-AND-6934, dated: 06/11/2020. Sankaran Balakrishnan worked on the project's concept, design, and monitoring and evaluation throughout the project. Sankaran Balakrishnan reviewed and edited the first draft of the paper, which was written by Geethanjali Kilari. Both the authors contributed to the final revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kilari, G., Balakrishnan, S. In Vitro Antiproliferative Activity and Phytochemicals Screening of Extracts of the Freshwater Microalgae, Chlorochromonas danica. Appl Biochem Biotechnol 195, 534–555 (2023). https://doi.org/10.1007/s12010-022-04137-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04137-7