Abstract
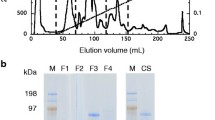
Second-generation biofuel production has emerged as a prominent sustainable and alternative energy. The biochemical properties of cellulolytic enzymes are imperative for cellulosic biomass conversion into fermentable sugars. In the present study, thermostable CMCase and β-glucosidase were purified and characterized from Aspergillus fumigatus JCM 10253. The enzymes were purified through 80% ammonium sulfate precipitation, followed by dialysis and DEAE-cellulose ion-exchange chromatography. The molecular masses of the purified CMCase and β-glucosidase were estimated to be 125 kDa and 90 kDa, respectively. The CMCase and β-glucosidase demonstrated optimum activities at pH 6.0 and 5.0, respectively. Their respective maximum temperatures were 50 and 60 °C. The cellulase activities were stimulated by 10 mM concentration of Ca2+, Ni2+, Fe2+, Mg2+, Cu2+, Mn2+, Zn2+, and Pb2+ ions. The CMCase activity was enhanced by surfactant Triton X-100 but marginally influenced by most inhibitors. The β-glucosidase retained its activity in the presence of organic solvents (30%) isoamyl alcohol, heptane, toluene, and ethyl acetate, while CMCase was retained with acetone during a prolonged incubation of 168 h. The Km and Vmax values of the two cellulases were studied. The properties of high thermostability and good tolerance against organic solvents could signify its potential use in biofuel production and other value-added products.






Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Gupta, R., Mehta, G., Deswal, D., Sharma, S., et al. (2013). Cellulases and their biotechnological applications. In R. C. Kuhad & A. Singh (Eds.), Biotechnology for Environmental Management and Resource Recovery (pp. 89–106). Springer.
Manavalan, T., Manavalan, A., Thangavelu, K. P., & Heese, K. (2015). Characterization of a novel endoglucanase from Ganoderma lucidum. Journal of basic Microbiology, 55(6), 761–771.
Tiwari, R., Nain, L., Labrou, N. E., & Shukla, P. (2018). Bioprospecting of functional cellulases from metagenome for second generation biofuel production: A review. Critical Reviews in Microbiology, 44(2), 244–257.
Zhao, C. H., Liu, X., Zhan, T., & He, J. (2018). Production of cellulase by Trichoderma reesei from pretreated straw and furfural residues. RSC advances, 8(63), 36233–36238.
Behera, B. C., Sethi, B. K., Mishra, R. R., Dutta, S. K., & Thatoi, H. N. (2017). Microbial cellulases–Diversity & biotechnology with reference to mangrove environment: A review. Journal of Genetic Engineering and Biotechnology, 15(1), 197–210.
Srivastava, N., Srivastava, M., Mishra, P. K., Gupta, V. K., Molina, G., Rodriguez-Couto, S., Manikanta, A., & Ramteke, P. W. (2018). Applications of fungal cellulases in biofuel production: Advances and limitations. Renewable and Sustainable Energy Reviews, 82, 2379–2386.
Rawat, R., Srivastava, N., Chadha, B. S., & Oberoi, H. S. (2014). Generating fermentable sugars from rice straw using functionally active cellulolytic enzymes from Aspergillus niger HO. Energy & Fuels, 28(8), 5067–5075.
Singhania, R. R., Patel, A. K., Sukumaran, R. K., Larroche, C., & Pandey, A. (2013). Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresource technology, 127, 500–507.
Singh, G., Verma, A.K. and Kumar, V., 2016. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech, 6(1), p.3.
Ramani, G., Meera, B., Vanitha, C., Rao, M., & Gunasekaran, P. (2012). Production, purification, and characterization of a β-glucosidase of Penicillium funiculosum NCL1. Applied biochemistry and biotechnology, 167(5), 959–972.
Yeoman, C. J., Han, Y., Dodd, D., Schroeder, C. M., Mackie, R. I., & Cann, I. K. (2010). Thermostable enzymes as biocatalysts in the biofuel industry. Advances in applied microbiology, 70, 1–55.
Budihal, S. R., Agsar, D., & Patil, S. R. (2016). Enhanced production and application of acidothermophilic Streptomyces cellulase. Bioresource technology, 200, 706–712.
Bisen, P.S. and Sharma, A., 2012. Introduction to instrumentation in life sciences. Crc Press
Kalsoom, R., Ahmed, S., Nadeem, M., Chohan, S., & Abid, M. (2018). Biosynthesis and extraction of cellulase produced by Trichoderma on agro-wastes. International Journal of Environmental Science and Technology, 16(2), 921–928.
Acharya, S., & Chaudhary, A. (2012). Bioprospecting thermophiles for cellulase production: A review. Brazilian Journal of Microbiology, 43(3), 844–856.
Srivastava, M., Srivastava, N., Ramteke, P.W. and Mishra, P.K. eds., 2019. Approaches to enhance industrial production of fungal cellulases. Springer.
Bhat, M. (2000). Cellulases and related enzymes in biotechnology. Biotechnology advances, 18(5), 355–383.
Singh, R.S., Singh, T. and Pandey, A., 2019. Microbial enzymes—An overview. Advances in Enzyme Technology, pp.1–40.
Phitsuwan, P., Laohakunjit, N., Kerdchoechuen, O., Kyu, K. L., & Ratanakhanokchai, K. (2013). Present and potential applications of cellulases in agriculture, biotechnology, and bioenergy. Folia microbiologica, 58(2), 163–176.
Kumar, V., Singh, D., Sangwan, P., & Gill, P. K. (2014). Global market scenario of industrial enzymes (pp. 173–196). Trends, scope and relevance. Nova Science Publishers, New York.
Singh, R., Kumar, M., Mittal, A. and Mehta, P.K., 2016. Microbial enzymes: Industrial progress in 21st century. 3 Biotech, 6(2), pp.1–15.
Islam, F., & Roy, N. (2018). Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC research notes, 11(1), 1–6.
Prasad, P., Singh, T. and Bedi, S., 2013. Characterization of the cellulolytic enzyme produced by Streptomyces griseorubens (Accession No. AB184139) isolated from Indian soil. Journal of King Saud University-Science, 25(3), pp.245–250.
Nguyen, K.A., Kumla, J., Suwannarach, N., Penkhrue, W. and Lumyong, S., 2019. Optimization of high endoglucanase yields production from polypore fungus, Microporus xanthopus strain KA038 under solid-state fermentation using green tea waste. Biology open, 8(11).
Ilyas, U., Gohar, F., Saeed, S., Bukhari, Z. and Ilyas, H., 2013. Screening of locally isolated Aspergillus species for their cellulolytic potential and their optimization on Vigna mungo in solid state fermentation. Biotechnology Journal International, pp.350–358.
Soliman, S.A., El-Zawahry, Y.A. and El-Mougith, A.A., 2013. Fungal biodegradation of agro-industrial waste. Cellulose-Biomass Conversion, pp.75–100.
Liu, D., Zhang, R., Yang, X., Xu, Y., Tang, Z., Tian, W., & Shen, Q. (2011). Expression, purification and characterization of two thermostable endoglucanases cloned from a lignocellulosic decomposing fungi Aspergillus fumigatus Z5 isolated from compost. Protein expression and purification, 79(2), 176–186.
Jiang, X., Du, J., He, R., Zhang, Z., Qi, F., Huang, J., & Qin, L. (2020). Improved production of majority cellulases in Trichoderma reesei by integration of cbh1 gene from Chaetomium thermophilum. Frontiers in microbiology, 11, 1633.
Lin, C., Shen, Z., & Qin, W. (2017). Characterization of xylanase and cellulase produced by a newly isolated Aspergillus fumigatus N2 and its efficient saccharification of barley straw. Applied biochemistry and Biotechnology, 182(2), 559–569.
Machado, A. S., Valadares, F., Silva, T. F., Milagres, A. M., Segato, F., & Ferraz, A. (2020). The secretome of Phanerochaete chrysosporium and Trametes versicolor grown in microcrystalline cellulose and use of the enzymes for hydrolysis of lignocellulosic materials. Frontiers in bioengineering and biotechnology, 8, 826.
Workman, W. E., & Day, D. F. (1982). Purification and properties of β-glucosidase from Aspergillus terreus. Applied and Environmental Microbiology, 44(6), 1289–1295.
Watanabe, T., & SATO, T., YOSHIOKA, S., KOSHIJIMA, T. and KUWAHARA, M. (1992). Purificication and properties of Aspergillus nigerβ-glucosidase. European Journal of Biochemistry, 209(2), 651–659.
Riou, C., Salmon, J. M., Vallier, M. J., Günata, Z., & Barre, P. (1998). Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Applied and Environmental Microbiology, 64(10), 3607–3614.
Liu, D., Zhang, R., Yang, X., Zhang, Z., Song, S., Miao, Y., & Shen, Q. (2012). Characterization of a thermostable β-glucosidase from Aspergillus fumigatus Z5, and its functional expression in Pichia pastoris X33. Microbial Cell Factories, 11(1), 1–15.
Saroj, P., Manasa, P., & Narasimhulu, K. (2018). Characterization of thermophilic fungi producing extracellular lignocellulolytic enzymes for lignocellulosic hydrolysis under solid-state fermentation. Bioresources and Bioprocessing, 5(1), 1–14.
Mandels, M. and Weber, J., 1969. The production of cellulases.
Saroj, P., Manasa, P., & Narasimhulu, K. (2021). Assessment and evaluation of cellulase production using ragi (Eleusine coracana) husk as a substrate from thermo-acidophilic Aspergillus fumigatus JCM 10253. Bioprocess and Biosystems Engineering, 44(1), 113–126.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257–268.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry, 31(3), 426–428.
Grover, A.K., MacMurchie, D.D. and Cushley, R.J., 1977. Studies on almond emulsin βd-glucosidase I. Isolation and characterization of a bifunctional isozyme. Biochimica et Biophysica Acta (BBA)-Enzymology, 482(1), pp.98–108.
Ghose, T.K. and Bisaria, V.S., 1987. Measurement of hemicellulase activities. Part 1: xylanases. Pure Appl Chem, 59(12), pp.1739–1751.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry, 72(1–2), 248–254.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature, 227(5259), pp.680–685.
Saqib, A. A. N., & Whitney, P. J. (2006). Esculin gel diffusion assay (EGDA): A simple and sensitive method for screening β-glucosidases. Enyzme and Microbial Technology, 39, 182–184.
Gohel, H. R., Contractor, C. N., Ghosh, S. K., & Braganza, V. J. (2013). A comparative study of various staining techniques for determination of extra cellular cellulase activity on carboxy methyl cellulose (CMC) agar plates. International Journal of Current Microbiology and Applied Sciences, 3(5), 261–266.
Pachauri, P., More, S., Sullia, S. B., & Deshmukh, S. (2017). Purification and characterization of cellulase from a novel isolate of Trichoderma longibrachiatum. Biofuels, 11(1), 85–91.
Decker, C., Visser, J. and Schreier, P., 2001. Β-glucosidase multiplicity from Aspergillus tubingensis CBS 643.92: Purification and characterization of four β-glucosidases and their differentiation with respect to substrate specificity, glucose inhibition and acid tolerance. Applied microbiology and biotechnology, 55(2), pp.157–163.
Asha, P., Divya, J., & Singh, I. B. (2016). Purification and characterisation of processive-type endoglucanase and β-glucosidase from Aspergillus ochraceus MTCC 1810 through saccharification of delignified coir pith to glucose. Bioresource technology, 213, 245–248.
Oh, J. M., Lee, J. P., Baek, S. C., Kim, S. G., Do Jo, Y., Kim, J., & Kim, H. (2018). Characterization of two extracellular β-glucosidases produced from the cellulolytic fungus Aspergillus sp. YDJ216 and their potential applications for the hydrolysis of flavone glycosides. International journal of biological macromolecules, 111, 595–603.
Narasimha, G., Sridevi, A., Ramanjaneyulu, G., & Rajasekhar Reddy, B. (2016). Purification and characterization of β-glucosidase from Aspergillus niger. International Journal of Food Properties, 19(3), 652–661.
Gao, L., Gao, F., Zhang, D., Zhang, C., Wu, G., & Chen, S. (2013). Purification and characterization of a new β-glucosidase from Penicillium piceum and its application in enzymatic degradation of delignified corn stover. Bioresource technology, 147, 658–661.
Pandey, A.K. and Negi, S., 2020. Enhanced cellulase recovery in SSF from Rhizopus oryzae SN5 and immobilization for multi-batch saccharification of carboxymethylcellulose. Biocatalysis and Agricultural Biotechnology, 26, p.101656.
Shruthi, B. R., Achur, R. N. H., & Nayaka Boramuthi, T. (2020). Optimized solid-state fermentation medium enhances the multienzymes production from Penicillium citrinum and Aspergillus clavatus. Current Microbiology, 77, 2192–2206.
Sulyman, A.O., Igunnu, A. and Malomo, S.O., 2020. Isolation, purification and characterization of cellulase produced by Aspergillus niger cultured on Arachis hypogaea shells. Heliyon, 6(12), p.e05668.
Bhanja, T., Rout, S., Banerjee, R., & Bhattacharyya, B. C. (2007). Comparative profiles of α-amylase production in conventional tray reactor and GROWTEK bioreactor. Bioprocess and biosystems engineering, 30(5), 369–376.
Tao, Y. M., Zhu, X. Z., Huang, J. Z., Ma, S. J., Wu, X. B., Long, M. N., & Chen, Q. X. (2010). Purification and properties of endoglucanase from a sugar cane bagasse hydrolyzing strain, Aspergillus glaucus XC9. Journal of Agricultural and Food Chemistry, 58(10), 6126–6130.
Liszka, M. J., Clark, M. E., Schneider, E., & Clark, D. S. (2012). Nature versus nurture: Developing enzymes that function under extreme conditions. Annual review of chemical and biomolecular engineering, 3, 77–102.
Shahriarinour, M., & Ramanan, R. N. (2015). Purification and characterisation of extracellular cellulase main components from Aspergillus terreus. BioResources, 10(3), 4886–4902.
Bano, A., Chen, X., Prasongsuk, S., Akbar, A., Lotrakul, P., Punnapayak, H., Anwar, M., Sajid, S., & Ali, I. (2019). Purification and characterization of cellulase from obligate halophilic Aspergillus flavus (TISTR 3637) and its prospects for bioethanol production. Applied biochemistry and biotechnology, 189(4), 1327–1337.
Santa-Rosa, P. S., Souza, A. L., Roque, R. A., Andrade, E. V., Astolfi-Filho, S., Mota, A. J., & Nunes-Silva, C. G. (2018). Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology, 31, 84–92.
Monteiro, L. M. O., Vici, A. C., Pinheiro, M. P., Heinen, P. R., de Oliveira, A. H. C., Ward, R. J., Prade, R. A., Buckeridge, M. S., & de Moraes, M. D. L. T. (2020). A highly glucose tolerant ß-glucosidase from Malbranchea pulchella (mp bg3) enables cellulose saccharification. Scientific reports, 10(1), 1–12.
Langston, J., Sheehy, N. and Xu, F., 2006. Substrate specificity of Aspergillus oryzae family 3 β-glucosidase. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1764(5), pp.972–978.
Silva, L. D. B., Gomes, T. C., Ullah, S. F., Ticona, A. R. P., Hamann, P. R. V., & Noronha, E. F. (2020). Biochemical properties of carbohydrate-active enzymes synthesized by Penicillium chrysogenum using corn straw as carbon source. Waste and Biomass Valorization, 11, 2455–2466.
Da Costa, S. G., Pereira, O. L., Teixeira-Ferreira, A., Valente, R. H., de Rezende, S. T., Guimarães, V. M., & Genta, F. A. (2018). Penicillium citrinum UFV1 β-glucosidases: Purification, characterization, and application for biomass saccharification. Biotechnology for biofuels, 11(1), 1–19.
Martins ME Da M., Martins E. Da S., Martins H.L., 2020. Production and characterization of a thermostable β-glucosidase from Myceliophthora heterothallica. Bioscience Journal, 36(1).
Pol, D., Laxman, R. S., & Rao, M. (2012). Purification and biochemical characterization of endoglucanase from Penicillium pinophilum MS 20. Indian Journal of Biochemistry & Biophysics, 49(3), 189–194.
Qin, Y., Li, Q., Luo, F., Fu, Y., & He, H. (2020). One-step purification of two novel thermotolerant β-1, 4-glucosidases from a newly isolated strain of Fusarium chlamydosporum HML278 and their characterization. AMB Express, 10(1), 1–11.
Alani, F., Anderson, W.A. and Moo-Young, M., 2008. New isolate of Streptomyces sp. with novel thermoalkalotolerant cellulases. Biotechnology letters, 30(1), pp.123–126.
Karami, F., Ghorbani, M., Mahoonak, A.S. and Khodarahmi, R., 2020. Fast, inexpensive purification of β-glucosidase from Aspergillus niger and improved catalytic/physicochemical properties upon the enzyme immobilization: Possible broad prospects for industrial applications. LWT, 118, p.108770.
Kudo, K., Watanabe, A., Ujiie, S., Shintani, T., & Gomi, K. (2015). Purification and enzymatic characterization of secretory glycoside hydrolase family 3 (GH3) aryl β-glucosidases screened from Aspergillus oryzae genome. Journal of bioscience and bioengineering, 120(6), 614–623.
Mrudula, S., & Murugammal, R. (2011). Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Brazilian Journal of Microbiology, 42(3), 1119–1127.
Gupta, R., Gigras, P., Mohapatra, H., Goswami, V. K., & Chauhan, B. (2003). Microbial α-amylases: A biotechnological perspective. Process biochemistry, 38(11), 1599–1616.
Pachauri, P., More, S., Aranganathan, V., & Sullia, S. B. (2018). Kinetic study and characterization of cellulase enzyme from isolated Aspergillus niger subsp. awamori for cellulosic biofuels. Journal of Scientific and Industrial Research, 77, 55–60.
Volkov, P. V., Rozhkova, A. M., Zorov, I. N., & Sinitsyn, A. P. (2020). Cloning, purification and study of recombinant GH3 family β-glucosidase from Penicillium verruculosum. Biochimie, 168, 231–240.
Yang, Y., Wang, J., Guo, H. and Cao, Y., 2020. The enzymatic characters of heterologous expressed novel β-1, 4-glucosidase originated from Aspergillus fresenii. 3 Biotech, 10, pp.1–9.
Pereira, J., de, C., Giese, E.C., Moretti, M.M., de, S., Gomes, A.C., dos, S., Perrone, O.M., Boscolo, M., Da Silva, R., Gomes, E., Martins, D.A.B., 2017. Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. In: Enzyme Inhibitors and Activators. InTech, pp. 139–164.
Okereke, O. E., Akanya, H. O., & Egwim, E. C. (2017). Purification and characterization of an acidophilic cellulase from Pleurotus ostreatus and its potential for agrowastes valorization. Biocatalysis and Agricultural Biotechnology, 12, 253–259.
Feng, T., Liu, H., Xu, Q., Sun, J., & Shi, H. (2015). Identification and characterization of two endogenous β-glucosidases from the termite Coptotermes formosanus. Applied biochemistry and biotechnology, 176(7), 2039–2052.
Stricks, W. and Kolthoff, I.M., 1953. Reactions between mercuric mercury and cysteine and glutathione. Apparent dissociation constants, heats and entropies of formation of various forms of mercuric mercapto-cysteine and-glutathione. Journal of the American Chemical Society, 75(22), pp.5673–5681.
Bonfá, E. C., de Souza Moretti, M. M., Gomes, E., & Bonilla-Rodriguez, G. O. (2018). Biochemical characterization of an isolated 50 kDa beta-glucosidase from the thermophilic fungus Myceliophthora thermophila M. 7.7. Biocatalysis and agricultural biotechnology, 13, 311–318.
Lee, H. J., Lee, Y. S., & Choi, Y. L. (2018). Cloning, purification, and characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221. Biotechnology for biofuels, 11(1), 1–14.
Bai, H., Wang, H., Junde Sun, M. I., Han, M., Huang, Y., Han, X., & Yang, Q. (2013). Production, purification and characterization of novel beta glucosidase from newly isolated Penicillium simplicissimum H-11 in submerged fermentation. EXCLI journal, 12, 528.
Gong, G., Zheng, Z., Liu, H., Wang, L., Diao, J., Wang, P., & Zhao, G. (2014). Purification and characterization of a beta-glucosidase from Aspergillus niger and its application in the hydrolysis of geniposide to genipin. Journal of Microbiology and Biotechnology, 24, 788–794.
Bansal, N., Janveja, C., Tewari, R., Soni, R., & Soni, S. K. (2014). Highly thermostable and pH-stable cellulases from Aspergillus niger NS-2: Properties and application for cellulose hydrolysis. Applied biochemistry and biotechnology, 172(1), 141–156.
Aich, S., Singh, R. K., Kundu, P., Pandey, S. P., & Datta, S. (2017). Genome-wide characterization of cellulases from the hemi-biotrophic plant pathogen, Bipolaris sorokiniana, reveals the presence of a highly stable GH7 endoglucanase. Biotechnology for biofuels, 10(1), 1–14.
Prajapati, B. P., Suryawanshi, R. K., Agrawal, S., Ghosh, M., & Kango, N. (2018). Characterization of cellulase from Aspergillus tubingensis NKBP-55 for generation of fermentable sugars from agricultural residues. Bioresource technology, 250, 733–740.
das Neves, C.A., de Menezes, L.H.S., Soares, G.A., dos Santos Reis, N., Tavares, I.M.C., Franco, M. and de Oliveira, J.R., 2020. Production and biochemical characterization of halotolerant β-glucosidase by Penicillium roqueforti ATCC 10110 grown in forage palm under solid-state fermentation. Biomass Conversion and Biorefinery, pp.1–12.
Souza, L. O., de Brito, A. R., Bonomo, R. C. F., Santana, N. B., Ferraz, J. L. A. A., Aguiar-Oliveira, E., & Franco, M. (2018). Comparison of the biochemical properties between the xylanases of Thermomyces lanuginosus (Sigma®) and excreted by Penicillium roqueforti ATCC 10110 during the solid state fermentation of sugarcane bagasse. Biocatalysis and Agricultural Biotechnology, 16, 277–284.
Ijaz, A., Anwar, Z., Irshad, M., Iqbal, Z., Arshad, M., Javed, M., & ZULFIQAR, M., AHMAD, A.R. and Ahmad, A. (2014). Purification and kinetic characterization of statistically optimized cellulase produced from Aspergillus niger. Romanian Biotechnological Letters, 19(6), 9836.
Akatin, M. Y. (2013). Characterization of a β-glucosidase from an edible mushroom, Lycoperdon pyriforme. International Journal of Food Properties, 16(7), 1565–1577.
Baffi, M. A., Martin, N., Tobal, T. M., Ferrarezi, A. L., Lago, J. H. G., Boscolo, M., Gomes, E., & Da-Silva, R. (2013). Purification and characterization of an ethanol-tolerant β-glucosidase from Sporidiobolus pararoseus and its potential for hydrolysis of wine aroma precursors. Applied biochemistry and biotechnology, 171(7), 1681–1691.
Arevalo-Villena, M., Ubeda Iranzo, J., & Briones Perez, A. (2007). Enhancement of aroma in white wines using a β-glucosidase preparation from Debaryomyces pseudopolymorphus (A-77). Food Biotechnology, 21(2), 181–194.
Liu, Y., Tan, H., Zhang, X., Yan, Y., & Hameed, B. H. (2010). Effect of monohydric alcohols on enzymatic transesterification for biodiesel production. Chemical Engineering Journal, 157(1), 223–229.
Wang, S., Meng, X., Zhou, H., Liu, Y., Secundo, F., & Liu, Y. (2016). Enzyme stability and activity in non-aqueous reaction systems: A mini review. Catalysts, 6(2), 32.
Su, E., & Wei, D. (2008). Improvement in lipase-catalyzed methanolysis of triacylglycerols for biodiesel production using a solvent engineering method. Journal of Molecular Catalysis B: Enzymatic, 55(3–4), 118–125.
Klibanov, A. M. (1990). Asymmetric transformations catalyzed by enzymes in organic solvents. Accounts of Chemical Research, 23(4), 114–120.
Mattos, C., & Ringe, D. (2001). Proteins in organic solvents. Current opinion in structural biology, 11(6), 761–764.
Sinha, R. and Khare, S.K., 2014. Effect of organic solvents on the structure and activity of moderately halophilic Bacillus sp. EMB9 protease. Extremophiles, 18(6), pp.1057–1066.
Kumar, A., Dhar, K., Kanwar, S. S., & Arora, P. K. (2016). Lipase catalysis in organic solvents: Advantages and applications. Biological Procedures Online, 18(1), 1–11.
Sonnleitner, B., & Fiechter, A. (1983). Advantages of using thermophiles in biotechnological processes: Expectations and reality. Trends in Biotechnology, 1(3), 74–80.
Cakmak, U., & Ertunga, N. S. (2016). Gene cloning, expression, immobilization and characterization of endo-xylanase from Geobacillus sp. TF16 and investigation of its industrial applications. Journal of Molecular Catalysis B: Enzymatic, 133, S288–S298.
Trinh, D.K., Quyen, D.T., Do, T.T. and Nghiem, N.M., 2013. Purification and characterization of a novel detergent-and organic solvent-resistant endo-beta-1, 4-glucanase from a newly isolated basidiomycete Peniophora sp. NDVN01. Turkish Journal of Biology, 37(4), pp.377–384.
Watanabe, A., Suzuki, M., Ujiie, S., & Gomi, K. (2016). Purification and enzymatic characterization of a novel β-1, 6-glucosidase from Aspergillus oryzae. Journal of bioscience and bioengineering, 121(3), 259–264.
Wang, Y., Li, J., & Xu, Y. (2011). Characterization of novel β-glucosidases with transglycosylation properties from Trichosporon asahii. Journal of agricultural and food chemistry, 59(20), 11219–11227.
Batra, J., & Mishra, S. (2013). Organic solvent tolerance and thermostability of a β-glucosidase co-engineered by random mutagenesis. Journal of Molecular Catalysis B: Enzymatic, 96, 61–66.
Piñuel, L., Breccia, J.D., Guisán, J.M. and López-Gallego, F., 2013. Production of hesperetin using a covalently multipoint immobilized diglycosidase from Acremonium sp. DSM24697. Journal of molecular microbiology and biotechnology, 23(6), pp.410–417.
Abdel-Fatah, O. M., Hassan, M. M., Elshafei, A. M., Haroun, B. M., Atta, H. M., & Othman, A. M. (2012). Physiological studies on carboxymethyl cellulase formation by Aspergillus terreus DSM 826. Brazilian journal of microbiology, 43(1), 01–11.
Prasanna, H.N., Ramanjaneyulu, G. and Reddy, B.R., 2016. Optimization of cellulase production by Penicillium sp. 3 Biotech, 6(2), pp.1–11.
Callow, N. V., & Ju, L. K. (2012). Promoting pellet growth of Trichoderma reesei Rut C30 by surfactants for easy separation and enhanced cellulase production. Enzyme and microbial technology, 50(6–7), 311–317.
Chan, C.S., Sin, L.L., Chan, K.G., Shamsir, M.S., Abd Manan, F., Sani, R.K. and Goh, K.M., 2016. Characterization of a glucose-tolerant β-glucosidase from Anoxybacillus sp. DT3–1. Biotechnology for biofuels, 9(1), pp.1–11.
Lucas, R., Robles, A., Alvarez de Cienfuegos, G., & Gálvez, A. (2000). β-Glucosidase from Chalara paradoxa CH32: Purification and properties. Journal of agricultural and food chemistry, 48(8), 3698–3703.
Wu, J., & Ju, L. K. (1998). Enhancing enzymatic saccharification of waste newsprint by surfactant addition. Biotechnology progress, 14(4), 649–652.
Asha, B. M., & Sakthivel, N. (2014). Production, purification and characterization of a new cellulase from Bacillus subtilis that exhibit halophilic, alkalophilic and solvent-tolerant properties. Annals of Microbiology, 64(4), 1839–1848.
Park, A. R., Hong, J. H., Kim, J. J., & Yoon, J. J. (2012). Biochemical characterization of an extracellular β-glucosidase from the fungus, Penicillium italicum, isolated from rotten citrus peel. Mycobiology, 40(3), 173–180.
Dave, B. R., Sudhir, A. P., & Subramanian, R. B. (2015). Purification and properties of an endoglucanase from Thermoascus aurantiacus. Biotechnology Reports, 6, 85–90.
Gudi, S. K., Gurramkonda, C., Chandra, S. M., Prasad, S. B. V., Yadav, S. P., Kumar, N. C. V. M., & Reddy, R. (2016). Thermostable β-D-glucosidase from Aspergillus flavus: Production, purification and characterization. International Journal of Clinical and Biological Sciences, 1, 1–15.
Scott, T. A. (1996). Concise encyclopedia biology. Walter de Gruyter.
Muensean, K., & Kim, S. M. (2015). Purification and characterization of β-glucosidase produced by Trichoderma citrinoviride cultivated on microalga Chlorella vulgaris. Applied biochemistry and microbiology, 51(1), 102–107.
Chen, Z., Liu, Y., Liu, L., Chen, Y., Li, S., & Jia, Y. (2019). Purification and characterization of a novel β-glucosidase from Aspergillus flavus and its application in saccharification of soybean meal. Preparative Biochemistry and Biotechnology, 49(7), 671–678.
Acknowledgements
The authors acknowledge the support provided by the Department of Biotechnology, National Institute of Technology Warangal, Telangana, India.
Author information
Authors and Affiliations
Contributions
Paramjeet Saroj performed the research experiments, data analysis, and writing of the manuscript. Manasa P contributed to the data collection and manuscript writing. Korrapati Narasimhulu supervised during research experiments and manuscript preparation. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saroj, P., P, M. & Narasimhulu, K. Biochemical Characterization of Thermostable Carboxymethyl Cellulase and β-Glucosidase from Aspergillus fumigatus JCM 10253. Appl Biochem Biotechnol 194, 2503–2527 (2022). https://doi.org/10.1007/s12010-022-03839-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03839-2