Abstract
Obesity, diabetes, and other cardiovascular diseases are directly related to the high consumption of processed sugars with high caloric content. The current food industry has novel trends related to replacing highly caloric sugars with non-caloric or low-calorie sweeteners. Mannitol, a polyol, represents a suitable substitute because it has a low caloric content and does not induce a glycemic response, which is crucial for diabetic people. Consequently, this polyol has multiple applications in the food, pharmaceutical, and medicine industries. Mannitol can be produced by plant extraction, chemical or enzymatic synthesis, or microbial fermentation. Different in vitro processes have been developed regarding enzymatic synthesis to obtain mannitol from fructose, glucose, or starch-derived substrates. Various microorganisms such as yeast, fungi, and bacteria are applied for microbial fermentation. Among them, heterofermentative lactic acid bacteria (LAB) represent a reliable and feasible alternative due to their metabolic characteristics. In this regard, the yield and productivity of mannitol depend on the culture system, the growing conditions, and the culture medium composition. In situ mannitol production represents a novel approach to decrease the sugar content in food and beverages. Also, genetic engineering offers an interesting option to obtain mannitol-producing strains. This review presents and discusses the most significant advances that have been made in the mannitol production through fermentation by heterofermentative LAB, including the pertinent and critical analysis of culture conditions considering broth composition, reaction systems, and their effects on productivities and yields.


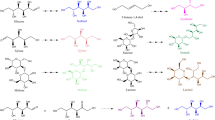
Modified from Wisselink et al. [5]

Modified from Wisselink et al. [5]

Modified from Sahin et al. [94]

Modified from Gaspar et al. [96]
Similar content being viewed by others
Data availability
Not applicable.
References
Akinterinwa, O., Khankal, R., & Cirino, P. C. (2008). Metabolic engineering for bioproduction of sugar alcohols. Current Opinion in Biotechnology, 19(5), 461–467. https://doi.org/10.1016/j.copbio.2008.08.002
Grembecka, M. (2015). Sugar alcohols—their role in the modern world of sweeteners: A review. European Food Research and Technology, 241(1), 1–14. https://doi.org/10.1007/s00217-015-2437-7
Park, Y. C., Oh, E. J., Jo, J. H., Jin, Y. S., & Seo, J. H. (2016). Recent advances in biological production of sugar alcohols. Current Opinion in Biotechnology, 37, 105–113. https://doi.org/10.1016/j.copbio.2015.11.006
Rice, T., Zannini, E., K. Arendt, E., & Coffey, A. (2020). A review of polyols–biotechnological production, food applications, regulation, labeling and health effects. Critical Reviews in Food Science and Nutrition, 60(12), 2034–2051. https://doi.org/10.1080/10408398.2019.1625859
Wisselink, H., Weusthuis, R., Eggink, G., Hugenholtz, J., & Grobben, G. (2002). Mannitol production by lactic acid bacteria: A review. International Dairy Journal, 12(2–3), 151–161. https://doi.org/10.1016/S0958-6946(01)00153-4
Martău, G. A., Coman, V., & Vodnar, D. C. (2020). Recent advances in the biotechnological production of erythritol and mannitol. Critical Reviews in Biotechnology, 40(5), 608–622. https://doi.org/10.1080/07388551.2020.1751057
Solomon, P. S., Waters, O. D. C., & Oliver, R. P. (2007). Decoding the mannitol enigma in filamentous fungi. Trends in Microbiology, 15(6), 257–262. https://doi.org/10.1016/j.tim.2007.04.002
Dai, Y., Meng, Q., Mu, W., & Zhang, T. (2017). Recent advances in the applications and biotechnological production of mannitol. Journal of Functional Foods, 36, 404–409. https://doi.org/10.1016/j.jff.2017.07.022
Patel, T. K., & Williamson, J. D. (2016). Mannitol in Plants, Fungi, and Plant-Fungal Interactions. Trends in Plant Science, 21(6), 486–497. https://doi.org/10.1016/j.tplants.2016.01.006
Grembecka, M. (2018). Sugar Alcohols as Sugar Substitutes in Food Industry. In J.-M. Mérillon & K. G. Ramawat (Eds.), Sweeteners: Pharmacology, Biotechnology and Applications (First., pp. 547–573). Switzerland: Springer. https://doi.org/10.1007/978-3-319-27027-2
Sylvetsky, A. C., & Rother, K. I. (2016). Trends in the consumption of low-calorie sweeteners. Physiology and Behavior, 164, 446–450. https://doi.org/10.1016/j.physbeh.2016.03.030
Williams, E. P., Mesidor, M., Winters, K., Dubbert, P. M., & Wyatt, S. B. (2015). Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Current obesity reports, 4(3), 363–370. https://doi.org/10.1007/s13679-015-0169-4
Ng, M., Fleming, T., Robinson, M., Thomson, B., Graetz, N., Margono, C., … Gakidou, E. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 384(9945), 766–781. https://doi.org/10.1016/S0140-6736(14)60460-8
Mooradian, A. D., Smith, M., & Tokuda, M. (2017). The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clinical Nutrition ESPEN, 18, 1–8. https://doi.org/10.1016/j.clnesp.2017.01.004
Agha, M., & Agha, R. (2017). The rising prevalence of obesity: Part A:Impact on public health. International Journal of Surgery Oncology, 2(7), 1–6. https://doi.org/10.1097/IJ9.0000000000000017
Chen, M., Zhang, W., Wu, H., Guang, C., & Mu, W. (2020). Mannitol: Physiological functionalities, determination methods, biotechnological production, and applications. Applied Microbiology and Biotechnology, 104(16), 6941–6951. https://doi.org/10.1007/s00253-020-10757-y
U.S. Government-FDA. (2020). Code of Federal Regulations -Title 21: Food and Drugs. Retrieved May 14, 2020, from https://www.ecfr.gov/cgi-bin/text-idx?SID=b4e358b1e7a8cc1f970a78df574da626&mc=true&node=se21.3.180_125&rgn=div8
Ortiz, M. E., Bleckwedel, J., Raya, R. R., & Mozzi, F. (2013). Biotechnological and in situ food production of polyols by lactic acid bacteria. Applied Microbiology and Biotechnology, 97(11), 4713–4726. https://doi.org/10.1007/s00253-013-4884-z
Saha, B. C., & Racine, F. M. (2011). Biotechnological production of mannitol and its applications. Applied Microbiology and Biotechnology, 89(4), 879–891. https://doi.org/10.1007/s00253-010-2979-3
Ohrem, H. L., Schornick, E., Kalivoda, A., & Ognibene, R. (2014). Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharmaceutical Development and Technology, 19(3), 257–262. https://doi.org/10.3109/10837450.2013.775154
Saffari, M., Ebrahimi, A., & Langrish, T. (2016). Nano-confinement of acetaminophen into porous mannitol through adsorption method. Microporous and Mesoporous Materials, 227, 95–103. https://doi.org/10.1016/j.micromeso.2016.02.047
Kulkarni, S. S., Suryanarayanan, R., Rinella, J. V., & Bogner, R. H. (2018). Mechanisms by which crystalline mannitol improves the reconstitution time of high concentration lyophilized protein formulations. European Journal of Pharmaceutics and Biopharmaceutics, 131, 70–81. https://doi.org/10.1016/j.ejpb.2018.07.022
André, P., & Villain, F. (2017). Free radical scavenging properties of mannitol and its role as a constituent of hyaluronic acid fillers: A literature review. International Journal of Cosmetic Science, 39(4), 355–360. https://doi.org/10.1111/ics.12386
Lugo-Baruqui, J. A., Ayyathurai, R., Sriram, A., & Pragatheeshwar, K. D. (2019). Use of Mannitol for Ischemia Reperfusion Injury in Kidney Transplant and Partial Nephrectomies—Review of Literature. Current Urology Reports, 20(1). https://doi.org/10.1007/s11934-019-0868-6
Backer, V. (2019). Mannitol and the mechanisms behind bronchoconstriction. Journal of Allergy and Clinical Immunology, 144(4), 931–932. https://doi.org/10.1016/j.jaci.2019.06.048
Ghoreishi, S. M., Shahrestani, G. R., & Ghaziaskar, H. S. (2009). Experimental and modeling investigation of supercritical extraction of mannitol from Olive leaves. Chemical Engineering and Technology, 32(1), 45–54. https://doi.org/10.1002/ceat.200800441
Ghoreishi, S. M., & Sharifi, S. (2001). Modeling of supercritical extraction of mannitol from plane tree leaf. Journal of Pharmaceutical and Biomedical Analysis, 24, 1037–1048. https://doi.org/10.1016/s0731-7085(00)00538-0
Ghoreishi, S. M., & Shahrestani, R. G. (2009). Subcritical water extraction of mannitol from olive leaves. Journal of Food Engineering, 93(4), 474–481. https://doi.org/10.1016/j.jfoodeng.2009.02.015
Lama-Muñoz, A., Contreras, M. del M., Espínola, F., Moya, M., Romero, I., & Castro, E. (2020). Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chemistry, 320, 126626. https://doi.org/10.1016/j.foodchem.2020.126626
Soetaert, W., Vanhooren, P. T., & Vandamme, E. J. (1999). The Production of Mannitol by Fermentation. In C. Bucke (Ed.), Carbohydrate Biotechnology Protocols (Vol. 10, pp. 261–275). Totowa, NJ: Humana Press Inc. https://doi.org/10.1007/978-1-59259-261-6_21
Zhang, M., Cheng, C., Jiang, M., Xin, F., Gu, L., Wu, H., … Liu, J. (2018). Recent advances in microbial production of mannitol: utilization of low-cost substrates, strain development and regulation strategies. World Journal of Microbiology and Biotechnology, 34(3), 0. https://doi.org/10.1007/s11274-018-2425-8
Kuusisto, J., Mikkola, J. P., Casal, P. P., Karhu, H., Väyrynen, J., & Salmi, T. (2005). Kinetics of the catalytic hydrogenation of D-fructose over a CuO-ZnO catalyst. Chemical Engineering Journal, 115(1–2), 93–102. https://doi.org/10.1016/j.cej.2005.09.020
Zelin, J., Meyer, C. I., Regenhardt, S. A., Sebastian, V., Garetto, T. F., & Marchi, A. J. (2017). Selective liquid-phase hydrogenation of fructose to D-mannitol over copper-supported metallic nanoparticles. Chemical Engineering Journal, 319, 48–56. https://doi.org/10.1016/j.cej.2017.02.127
Zelin, J., Regenhardt, S. A., Meyer, C. I., Duarte, H. A., Sebastian, V., & Marchi, A. J. (2019). Selective aqueous-phase hydrogenation of D-fructose into D-mannitol using a highly efficient and reusable Cu-Ni/SiO2 catalyst. Chemical Engineering Science, 206, 315–326. https://doi.org/10.1016/j.ces.2019.05.042
Lu, F., Xu, W., Zhang, W., Guang, C., & Mu, W. (2019). Polyol dehydrogenases: Intermediate role in the bioconversion of rare sugars and alcohols. Applied Microbiology and Biotechnology, 103(16), 6473–6481. https://doi.org/10.1007/s00253-019-09980-z
Liu, S., Saha, B., & Cotta, M. (2005). Cloning, Expression, Purification, and Analysis of Mannitol Dehydrogenase Gene mtlK from Lactobacillus brevis. Applied Biochemistry and Biotechnology - Part A Enzyme Engineering and Biotechnology, 121(1–3), 391–401. https://doi.org/10.1007/978-1-59259-991-2_34
Kulbe, K. D., Schwab, U., & Gudernatsch, W. (1987). Enzyme-Catalyzed Production of Mannitol and Gluconic Acid. Annals of the New York Academy of Sciences, 506, 552–568. https://doi.org/10.1111/j.1749-6632.1987.tb23850.x
Slatner, M., Nagl, G., Haltrich, D., Kulbe, K. D., & Nidetzky, B. (1998). Enzymatic production of pure D-mannitol at high productivity. Biocatalysis and Biotransformation, 16(5), 351–363. https://doi.org/10.3109/10242429809003628
Parmentier, S., Arnaut, F., Soetaert, W., & Vandamme, E. J. (2005). Enzymatic production of D-mannitol with the Leuconostoc pseudomesenteroides mannitol dehydrogenase coupled to a coenzyme regeneration system. Biocatalysis and Biotransformation, 23(1), 1–7. https://doi.org/10.1080/10242420500071664
Maria, G. (2020). Model-based optimisation of a batch reactor with a coupled bi-enzymatic process for mannitol production. Computers and Chemical Engineering, 133, 1–7. https://doi.org/10.1016/j.compchemeng.2019.106628
Xu, W., Lu, F., Wu, H., Zhang, W., & Guang, C. (2020). Identification of a highly thermostable mannitol 2-dehydrogenase from Caldicellulosiruptor morganii Rt8.B8 and its application for the preparation of D-mannitol. Process Biochemistry, 96, 194–201. https://doi.org/10.1016/j.procbio.2020.05.014
Koko, M. Y. F., Mu, W., Hassanin, H. A. M., Zhang, S., Lu, H., Mohammed, J. K., … Yang, L. (2020). Archaeal hyperthermostable mannitol dehydrogenases: A promising industrial enzymes for D-mannitol synthesis. Food Research International, 137, 109638. https://doi.org/10.1016/j.foodres.2020.109638
Wei, X., Li, Q., Hu, C., & You, C. (2021). An ATP-free in vitro synthetic enzymatic biosystem facilitating one-pot stoichiometric conversion of starch to mannitol. Applied Microbiology and Biotechnology, 105(5), 1913–1924. https://doi.org/10.1007/s00253-021-11154-9
Fontes, C. P. M. L., Honorato, T. L., Rabelo, M. C., & Rodrigues, S. (2009). Kinetic study of mannitol production using cashew apple juice as substrate. Bioprocess and Biosystems Engineering, 32(4), 493–499. https://doi.org/10.1007/s00449-008-0269-6
Song, K. H., Lee, J. K., Song, J. Y., Hong, S. G., Baek, H., Kim, S. Y., & Hyun, H. H. (2002). Production of mannitol by a novel strain of Candida magnoliae. Biotechnology Letters, 24(1), 9–12. https://doi.org/10.1023/A:1013824309263
Lee, J. K., Song, J. Y., & Kim, S. Y. (2003). Controlling substrate concentration in fed-batch Candida magnoliae culture increases mannitol production. Biotechnology Progress, 19(3), 768–775. https://doi.org/10.1021/bp034025o
Baek, H., Song, K. H., Park, S. M., Kim, S. Y., & Hyun, H. H. (2003). Role of glucose in the bioconversion of fructose into mannitol by Candida magnoliae. Biotechnology Letters, 25(10), 761–765. https://doi.org/10.1023/A:1023512218206
Meng, Q., Zhang, T., Wei, W., Mu, W., & Miao, M. (2017). Production of Mannitol from a High Concentration of Glucose by Candida parapsilosis SK26.001. Applied Biochemistry and Biotechnology, 181(1), 391–406. https://doi.org/10.1007/s12010-016-2219-0
Khan, A., Bhide, A., & Gadre, R. (2009). Mannitol production from glycerol by resting cells of Candida magnoliae. Bioresource Technology, 100(20), 4911–4913. https://doi.org/10.1016/j.biortech.2009.04.048
Tomaszewska, L., Rywińska, A., & Gladkowski, W. (2012). Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. Journal of Industrial Microbiology and Biotechnology, 39(9), 1333–1343. https://doi.org/10.1007/s10295-012-1145-6
Yoshikawa, J., Habe, H., Morita, T., Fukuoka, T., Imura, T., Iwabuchi, H., … Kitamoto, D. (2014). Production of mannitol from raw glycerol by Candida azyma. Journal of Bioscience and Bioengineering, 117(6), 725–729. https://doi.org/10.1016/j.jbiosc.2013.11.016
Rakicka-Pustułka, M., Miedzianka, J., Jama, D., Kawalec, S., Liman, K., Janek, T., … Lazar, Z. (2021). High value-added products derived from crude glycerol via microbial fermentation using Yarrowia clade yeast. Microbial Cell Factories, 20(1), 1–18. https://doi.org/10.1186/s12934-021-01686-0
Vélëz, H., Glassbrook, N. J., & Daub, M. E. (2007). Mannitol metabolism in the phytopathogenic fungus Alternaria alternata. Fungal Genetics and Biology, 44(4), 258–268. https://doi.org/10.1016/j.fgb.2006.09.008
Ruijter, G. J. G., Visser, J., & Rinzema, A. (2004). Polyol accumulation by Aspergillus oryzae at low water activity in solid-state fermentation. Microbiology, 150(4), 1095–1101. https://doi.org/10.1099/mic.0.26723-0
Teertstra, W. R., Tegelaar, M., Dijksterhuis, J., Golovina, E. A., Ohm, R. A., & Wösten, H. A. B. (2017). Maturation of conidia on conidiophores of Aspergillus niger. Fungal Genetics and Biology, 98, 61–70. https://doi.org/10.1016/j.fgb.2016.12.005
Duan, R., Li, H., Li, H., Tang, L., Zhou, H., Yang, X., … Ding, Z. (2018). Enhancing the Production of D-Mannitol by an Artificial Mutant of Penicillium sp. T2-M10. Applied Biochemistry and Biotechnology, 186(4), 990–998. https://doi.org/10.1007/s12010-018-2791-6
De Vos, W. M. (2011). Systems solutions by lactic acid bacteria: From paradigms to practice. Microbial Cell Factories, 10(1), 1–13. https://doi.org/10.1186/1475-2859-10-S1-S2
Sauer, M., Russmayer, H., Grabherr, R., Peterbauer, C. K., & Marx, H. (2017). The Efficient Clade: Lactic Acid Bacteria for Industrial Chemical Production. Trends in Biotechnology, 35(8), 756–769. https://doi.org/10.1016/j.tibtech.2017.05.002
Hatti-Kaul, R., Chen, L., Dishisha, T., & Enshasy, H. E. (2018). Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS Microbiology Letters, 365(20), 1–20. https://doi.org/10.1093/femsle/fny213
De Man, J. C., Rogosa, M., & Sharpe, M. E. (1960). A medium for the cultivation of Lactobacilli. Journal of Applied Bacteriology, 23(1), 130–135. https://doi.org/10.1111/j.1365-2672.1960.tb00188.x
Årsköld, E., Lohmeier-Vogel, E., Cao, R., Roos, S., Rådström, P., & Van Niel, E. W. J. (2008). Phosphoketolase pathway dominates in Lactobacillus reuteri ATCC 55730 containing dual pathways for glycolysis. Journal of Bacteriology, 190(1), 206–212. https://doi.org/10.1128/JB.01227-07
Burgé, G., Saulou-Bérion, C., Moussa, M., Allais, F., Athes, V., & Spinnler, H. E. (2015). Relationships between the use of Embden Meyerhof pathway (EMP) or Phosphoketolase pathway (PKP) and lactate production capabilities of diverse Lactobacillus reuteri strains. Journal of Microbiology, 53(10), 702–710. https://doi.org/10.1007/s12275-015-5056-x
Song, S. H., & Vieille, C. (2009). Recent advances in the biological production of mannitol. Applied Microbiology and Biotechnology, 84(1), 55–62. https://doi.org/10.1007/s00253-009-2086-5
Gänzle, M. G. (2015). Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Current Opinion in Food Science, 2, 106–117. https://doi.org/10.1016/j.cofs.2015.03.001
Monedero, V., Pérez-Martínez, G., & Yebra, M. J. (2010). Perspectives of engineering lactic acid bacteria for biotechnological polyol production. Applied Microbiology and Biotechnology, 86(4), 1003–1015. https://doi.org/10.1007/s00253-010-2494-6
Yun, J. W., & Kim, D. H. (1998). A Comparative Study of Mannitol Production by Two Lactic Acid Bacteria. Journal of Fermentation and Bioengineering, 85(2), 203–208. https://doi.org/10.1016/S0922-338X(97)86768-2
Erten, H. (1998). Metabolism of fructose as an electron acceptor by Leuconostoc mesenteroides. Process Biochemistry, 33(7), 735–739. https://doi.org/10.1016/S0032-9592(98)00041-7
Grobben, G. J., Peters, S. W. P. G., Wisselink, H. W., Weusthuis, R. A., Hoefnagel, M. H. N., Hugenholtz, J., & Eggink, G. (2001). Spontaneous Formation of a Mannitol-Producing Variant of Leuconostoc pseudomesenteroides Grown in the Presence of Fructose. Applied and Environmental Microbiology, 67(6), 2867–2870. https://doi.org/10.1128/AEM.67.6.2867
von Weymarn, N., Hujanen, M., & Leisola, M. (2002). Production of D-mannitol by heterofermentative lactic acid bacteria. Process Biochemistry, 37(11), 1207–1213. https://doi.org/10.1016/S0032-9592(01)00339-9
von Weymarn, N., Kiviharju, K., & Leisola, M. (2002). High-level production of D-mannitol with membrane cell-recycle bioreactor. Journal of Industrial Microbiology and Biotechnology, 29(1), 44–49. https://doi.org/10.1038/sj.jim.7000262
von Weymarn, N., Kiviharju, K. J., Jääskeläinen, S. T., & Leisola, M. S. A. (2003). Scale-up of a new bacterial mannitol production process. Biotechnology Progress, 19(3), 815–821. https://doi.org/10.1021/bp025718s
Saha, B. C., & Nakamura, L. K. (2003). Production of mannitol and lactic acid by fermentation with Lactobacillus intermedius NRRL B-3693. Biotechnology and Bioengineering, 82(7), 864–871. https://doi.org/10.1002/bit.10638
Rodríguez, C., Rimaux, T., Fornaguera, M. J., Vrancken, G., De Valdez, G. F., De Vuyst, L., & Mozzi, F. (2012). Mannitol production by heterofermentative Lactobacillus reuteri CRL 1101 and Lactobacillus fermentum CRL 573 in free and controlled pH batch fermentations. Applied Microbiology and Biotechnology, 93(6), 2519–2527. https://doi.org/10.1007/s00253-011-3617-4
Yue, M., Cao, H., Zhang, J., Li, S., Meng, Y., Chen, W., … Du, Y. (2013). Improvement of mannitol production by Lactobacillus brevis mutant 3-A5 based on dual-stage pH control and fed-batch fermentations. World Journal of Microbiology and Biotechnology, 29(10), 1923–1930. https://doi.org/10.1007/s11274-013-1357-6
Tyler, C. A., Kopit, L., Doyle, C., Yu, A. O., Hugenholtz, J., & Marco, M. L. (2016). Polyol production during heterofermentative growth of the plant isolate Lactobacillus florum 2F. Journal of Applied Microbiology, 120(5), 1336–1345. https://doi.org/10.1111/jam.13108
Behare, P. V., Mazhar, S., Pennone, V., & McAuliffe, O. (2020). Evaluation of lactic acid bacteria strains isolated from fructose-rich environments for their mannitol-production and milk-gelation abilities. Journal of Dairy Science, 103(12), 11138–11151. https://doi.org/10.3168/jds.2020-19120
Saha, B. C. (2006). A low-cost medium for mannitol production by Lactobacillus intermedius NRRL B-3693. Applied Microbiology and Biotechnology, 72(4), 676–680. https://doi.org/10.1007/s00253-006-0364-z
Saha, B. C. (2006). Effect of salt nutrients on mannitol production by Lactobacillus intermedius NRRL B-3693. Journal of Industrial Microbiology and Biotechnology, 33(10), 887–890. https://doi.org/10.1007/s10295-006-0140-1
Saha, B. C. (2006). Production of mannitol from inulin by simultaneous enzymatic saccharification and fermentation with Lactobacillus intermedius NRRL B-3693. Enzyme and Microbial Technology, 39(5), 991–995. https://doi.org/10.1016/j.enzmictec.2006.02.001
Racine, F. M., & Saha, B. C. (2007). Production of mannitol by Lactobacillus intermedius NRRL B-3693 in fed-batch and continuous cell-recycle fermentations. Process Biochemistry, 42(12), 1609–1613. https://doi.org/10.1016/j.procbio.2007.09.001
Saha, B. C., & Racine, F. M. (2010). Effects of pH and corn steep liquor variability on mannitol production by Lactobacillus intermedius NRRL B-3693. Applied Microbiology and Biotechnology, 87(2), 553–560. https://doi.org/10.1007/s00253-010-2552-0
Ortiz, M. E., Fornaguera, M. J., Raya, R. R., & Mozzi, F. (2012). Lactobacillus reuteri CRL 1101 highly produces mannitol from sugarcane molasses as carbon source. Applied Microbiology and Biotechnology, 95(4), 991–999. https://doi.org/10.1007/s00253-012-3945-z
Ortiz, M. E., Raya, R. R., & Mozzi, F. (2015). Efficient mannitol production by wild-type Lactobacillus reuteri CRL 1101 is attained at constant pH using a simplified culture medium. Applied Microbiology and Biotechnology, 99(20), 8717–8729. https://doi.org/10.1007/s00253-015-6730-y
Fontes, C. P. M. L., Santiago Silveira, M., Guilherme, A. A., Fernandes, F. A. N., & Rodrigues, S. (2013). Substitution of yeast extract by ammonium sulfate for mannitol production in cashew apple juice. Biocatalysis and Agricultural Biotechnology, 2(1), 69–75. https://doi.org/10.1016/j.bcab.2012.11.003
Carvalheiro, F., Moniz, P., Duarte, L. C., Esteves, M. P., & Gírio, F. M. (2011). Mannitol production by lactic acid bacteria grown in supplemented carob syrup. Journal of Industrial Microbiology and Biotechnology, 38(1), 221–227. https://doi.org/10.1007/s10295-010-0823-5
Zhang, M., Gu, L., Cheng, C., Zhu, J., Wu, H., Ma, J., … Ouyang, P. (2017). High-yield production of mannitol by Leuconostoc pseudomesenteroides CTCC G123 from chicory-derived inulin hydrolysate. Journal of Industrial Microbiology and Biotechnology, 44(8), 1237–1244. https://doi.org/10.1007/s10295-017-1953-9
Cao, H., Yue, M., Liu, G., Du, Y., & Yin, H. (2018). Microbial production of mannitol by Lactobacillus brevis 3–A5 from concentrated extract of Jerusalem artichoke tubers. Biotechnology and Applied Biochemistry, 65(3), 484–489. https://doi.org/10.1002/bab.1590
Hijosa-Valsero, M., Garita-Cambronero, J., Paniagua-García, A. I., & Díez-Antolínez, R. (2021). Mannitol bioproduction from surplus grape musts and wine lees. LWT, 151, 112083. https://doi.org/10.1016/j.lwt.2021.112083
Sahin, A. W., Rice, T., Zannini, E., Axel, C., Coffey, A., Lynch, K. M., & Arendt, E. K. (2019). Leuconostoc citreum TR116: In-situ production of mannitol in sourdough and its application to reduce sugar in burger buns. International Journal of Food Microbiology, 302, 80–89. https://doi.org/10.1016/j.ijfoodmicro.2018.06.026
Rice, T., Sahin, A. W., Lynch, K. M., Arendt, E. K., & Coffey, A. (2020). Isolation, characterisation and exploitation of lactic acid bacteria capable of efficient conversion of sugars to mannitol. International Journal of Food Microbiology, 321, 108546. https://doi.org/10.1016/j.ijfoodmicro.2020.108546
Ma, S., Wang, Z., Guo, X., Wang, F., Huang, J., Sun, B., & Wang, X. (2021). Sourdough improves the quality of whole-wheat flour products: Mechanisms and challenges—A review. Food Chemistry, 360, 130038. https://doi.org/10.1016/j.foodchem.2021.130038
Gobbetti, M., De Angelis, M., Di Cagno, R., Calasso, M., Archetti, G., & Rizzello, C. G. (2019). Novel insights on the functional/nutritional features of the sourdough fermentation. International Journal of Food Microbiology, 302, 103–113. https://doi.org/10.1016/j.ijfoodmicro.2018.05.018
De Vuyst, L., Comasio, A., & Kerrebroeck, S. Van. (2021). Sourdough production: fermentation strategies, microbial ecology, and use of non-flour ingredients. Critical Reviews in Food Science and Nutrition, 0, 1–33. https://doi.org/10.1080/10408398.2021.1976100
Sahin, A. W., Rice, T., Zannini, E., Lynch, K. M., Coffey, A., & Arendt, E. K. (2019). The incorporation of sourdough in sugar-reduced biscuits: A promising strategy to improve techno-functional and sensory properties. European Food Research and Technology, 245(9), 1841–1854. https://doi.org/10.1007/s00217-019-03302-3
Chen, C., Lu, Y., Yu, H., Chen, Z., & Tian, H. (2019). Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Bioscience, 27, 30–36. https://doi.org/10.1016/j.fbio.2018.11.006
Gaspar, P., Neves, A. R., Gasson, M. J., Shearman, C. A., & Santos, H. (2011). High yields of 2,3-butanediol and mannitol in Lactococcus lactis through engineering of NAD+ cofactor recycling. Applied and Environmental Microbiology, 77(19), 6826–6835. https://doi.org/10.1128/AEM.05544-11
Wisselink, H. W., Moers, A. P. H. A., Mars, A. E., Hoefnagel, M. H. N., De Vos, W. M., & Hugenholtz, J. (2005). Overproduction of heterologous mannitol 1-phosphatase: A key factor for engineering mannitol production by Lactococcus lactis. Applied and Environmental Microbiology, 71(3), 1507–1514. https://doi.org/10.1128/AEM.71.3.1507-1514.2005
Xiao, H., Wang, Q., Bang-Berthelsen, C. H., Jensen, P. R., & Solem, C. (2020). Harnessing Adaptive Evolution to Achieve Superior Mannitol Production by Lactococcus lactis Using Its Native Metabolism. Journal of Agricultural and Food Chemistry, 68(17), 4912–4921. https://doi.org/10.1021/acs.jafc.0c00532
Xiao, H., Bang-Berthelsen, C. H., Jensen, P. R., & Solem, C. (2021). Deciphering the Regulation of the Mannitol Operon Paves the Way for Efficient Production of Mannitol in Lactococcus lactis. Applied and Environmental Microbiology, 87(16), 1–14. https://doi.org/10.1128/AEM.00779-21
Zhang, Z., Cheng, W. Y., Ju, X. Y., & Jin, H. X. (2014). The Effect of Dextransucrase Gene Inactivation on Mannitol Production by Leuconostoc mesenteroides. Indian Journal of Microbiology, 55(1), 35–40. https://doi.org/10.1007/s12088-014-0503-7
Papagianni, M., & Legiša, M. (2014). Increased mannitol production in Lactobacillus reuteri ATCC 55730 production strain with a modified 6-phosphofructo-1-kinase. Journal of Biotechnology, 181, 20–26. https://doi.org/10.1016/j.jbiotec.2014.04.007
Kaup, B., Bringer-Meyer, S., & Sahm, H. (2004). Metabolic engineering of Escherichia coli: Construction of an efficient biocatalyst for D-mannitol formation in a whole-cell biotransformation. Applied Microbiology and Biotechnology, 64(3), 333–339. https://doi.org/10.1007/s00253-003-1470-9
Heuser, F., Marin, K., Kaup, B., Bringer, S., & Sahm, H. (2009). Improving D-mannitol productivity of Escherichia coli: Impact of NAD, CO2 and expression of a putative sugar permease from Leuconostoc pseudomesenteroides. Metabolic Engineering, 11(3), 178–183. https://doi.org/10.1016/j.ymben.2009.01.006
Reshamwala, S. M. S., Pagar, S. K., Velhal, V. S., Maranholakar, V. M., Talangkar, V. G., & Lali, A. M. (2014). Construction of an efficient Escherichia coli whole-cell biocatalyst for D-mannitol production. Journal of Bioscience and Bioengineering, 118(6), 628–631. https://doi.org/10.1016/j.jbiosc.2014.05.004
Madsen, M. A., Semerdzhiev, S., Amtmann, A., & Tonon, T. (2018). Engineering Mannitol Biosynthesis in Escherichia coli and Synechococcus sp. PCC 7002 Using a Green Algal Fusion Protein. ACS Synthetic Biology, 7(12), 2833–2840. https://doi.org/10.1021/acssynbio.8b00238
Sánchez, C. (2009). Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnology Advances, 27(2), 185–194. https://doi.org/10.1016/j.biotechadv.2008.11.001
Singh, N., & Gaur, S. (2021). GRAS Fungi: A New Horizon in Safer Food Product. In X. Dai, M. Sharma, & J. Chen (Eds.), Fungi in Sustainable Food Production (First., pp. 27–37). Springer. https://doi.org/10.1007/978-3-030-64406-2_3
Funding
This work was supported by the Instituto Politécnico Nacional (Mexico) through the research project SIP-20211198. JGMM thanks the Consejo Nacional de Ciencia y Tecnología (CONACYT, Mexico) for Master Fellowship support (grant number: 735208).
Author information
Authors and Affiliations
Contributions
JGMM: original idea, conceptualization, literature search and data analysis, original draft. IC: critical revision of the work and editing. EDP: critical revision of the work and editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors have no competing interests to declare relevant to this article's content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martínez-Miranda, J.G., Chairez, I. & Durán-Páramo, E. Mannitol Production by Heterofermentative Lactic Acid Bacteria: a Review. Appl Biochem Biotechnol 194, 2762–2795 (2022). https://doi.org/10.1007/s12010-022-03836-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03836-5