Abstract
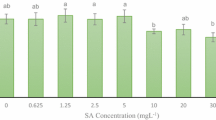
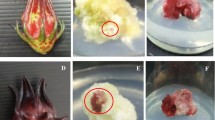
Allium jesdianum Boiss. & Buhse. is the most important species of the Amaryllidaceae family with various pharmacological properties. Three subsequent experiments (germination, callogenesis, and elicitation) were carried out as a completely randomized design with six replication. At the first study, the highest seed germination (78.33%) was achieved at chemical pre-treatment including the combination of α-naphthalene acetic acid (1 mg L−1) and benzylaminopurine (3 mg L−1) under in vitro condition. The highest callus induction (86.7%) was observed at MS/2 media, which was supplemented by NAA (1 mg L−1) and BAP (3 mg L−1) from hypocotyl explants. Then, two chemical elicitors including methyl jasmonate (MeJ) (0, 25, 50, and 100 µM) and putrescine (Pu) (0, 0.5, and 1 mM) were used to investigate their effects on different biochemical traits under callus culture. The results showed the superiority of MeJ over Pu for increasing the secondary metabolites and antioxidant activity in calluses of Allium jesdianum, compared to the control. The highest contents for total phenolics (6.02 mg GAE g−1 FW), total flavonoids (0.52 mg QE g−1 FW), and total flavonols (0.39 mg QE g−1 FW) were observed under 50 µM of MeJ. Meanwhile, the highest value for anthocyanin (8.99 µ mol g−1 FW) was achieved at 25 µM of MeJ. The highest 2,2-diphenyl-1-picrylhydrazyl activities were observed at 50 and 100 µM of MeJ. Putrescine (0.5 mM) elicitation showed only superiority for callus growth rate (0.53 mm day−1). Enhancement of desired secondary metabolites at 50 µM MeJ could be suitable for future studies in biotechnological aspects of this medicinal plant.

Similar content being viewed by others
Availability of Data and Materials
No applicable.
References
Yue, W., Ming, Q., Lin, B., Rahman, K., Zheng, C. J., Han, T., & Qin, L. P. (2016). Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Critical Reviews in Biotechnology, 36(2), 215–232.
Namdeo, A. G. (2007). Plant cell elicitation for production of secondary metabolites: A review. Pharmacognosy Reviews, 1(1), 69–79.
Erb, M., & Kliebenstein, D. J. (2020). Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiology, 184(1), 39–52.
Smetanska, I. (2008). Production of secondary metabolites using plant cell cultures. Food Biotechnology, 111, 187–228.
Vanisree, M., Lee, C. Y., Lo, S. F., Nalawade, S. M., Lin, C. Y., & Tsay, H. S. (2004). Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Botanical Bulletin of Academia Sinica, 45(1), 1–22.
Dias, M. I., Sousa, M. J., Alves, R. C., & Ferreira, I. C. (2016). Exploring plant tissue culture to improve the production of phenolic compounds: A review. Industrial Crops and Products, 82, 9–22.
Matkowski, A. (2008). Plant in vitro culture for the production of antioxidants-a review. Biotechnology Advances, 26(6), 548–560.
Narayani, M., & Srivastava, S. (2017). Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochemistry Reviews, 16(6), 1227–1252.
Thiruvengadam, M., Rekha, K., Rajakumar, G., Lee, T. J., Kim, S. H., & Chung, I. M. (2016). Enhanced production of anthraquinones and phenolic compounds and biological activities in the cell suspension cultures of Polygonum multiflorum. International Journal of Molecular Sciences, 17(11), 1912.
Nabi, N., Singh, S., & Saffeullah, P. (2021). Responses of in vitro cell cultures to elicitation: regulatory role of jasmonic acid and methyl jasmonate: a review. In Vitro Cellular and Developmental Biology - Plant, 1–15.
Singh, A., & Dwivedi, P. (2018). Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. Journal of Pharmacognosy Phytochemistry, 7(1), 750–757.
Gabr, A. M., Ghareeb, H., El Shabrawi, H. M., Smetanska, I., & Bekheet, S. A. (2016). Enhancement of silymarin and phenolic compound accumulation in tissue culture of milk thistle using elicitor feeding and hairy root cultures. Journal of Genetic Engineering and Biotechnology, 14(2), 327–333.
Ho, T. T., Murthy, H. N., & Park, S. Y. (2020). Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. International Journal of Molecular Sciences, 21(3), 716.
Mendoza, D., Arias, J. P., Cuaspud, O., Ruiz, O., & Arias, M. (2018). Methyl jasmonate/salicylic acid enhanced flavonoid production and change metabolites profile in Thevetia peruviana cell culture. In Vitro Cellular and Developmental Biology- Plant, 54, 4–27.
Chen, D., Shao, Q., Yin, L., Younis, A., & Zheng, B. (2019). Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Frontiers in Plant Science, 9, 1945.
Alcazar, R., Bueno, M., & Tiburcio, A. F. (2020). Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells, 9(11), 2373.
Jimenez Bremont, J. F., Marina, M., Guerrero-Gonzalez, M. D. L. L., Rossi, F. R., Sanchez-Rangel, D., Rodriguez-Kessler, M., Rui, O. A., & Garriz, A. (2014). Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Frontiers in Plant Science, 5, 95.
Rangan, P., Subramani, R., Kumar, R., Singh, A. K., & Singh, R. (2014). Recent advances in polyamine metabolism and abiotic stress tolerance. BioMed Research International, 2014, 1–9.
Rakesh, B., Sudheer, W. N., & Nagella, P. (2021). Role of polyamines in plant tissue culture: An overview. Plant Cell Tissue and Organ Culture, 1–20.
Mustafavi, S. H., Badi, H. N., Sękara, A., Mehrafarin, A., Janda, T., Ghorbanpour, M., & Rafiee, H. (2018). Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiologiae Plantarum, 40, 102.
Thiruvengadam, M., & Chung, I. M. (2015). Phenolic compound production and biological activities from in vitro regenerated plants of gherkin (Cucumis anguria L.). Electronic Journal of Biotechnology, 18(4), 295–301.
Li, Z., & Burritt, D. J. (2003). Changes in endogenous polyamines during the formation of somatic embryos from isogenic lines of Dactylis glomerata L. with different regenerative capacities. Plant Growth Regulation, 40, 65–74.
Klimek-Chodacka, M., Kadluczka, D., Lukasiewicz, A., Malec-Pala, A., Baranski, R., & Grzebelus, E. (2020). Effective callus induction and plant regeneration in callus and protoplast cultures of Nigella damascena L. Plant Cell Tissue and Organ Culture, 143(3), 693–707.
Fraguas, C. B., Villa, F., & Lima, G. P. P. (2009). Evaluation of exogenous application of polyamines on callus growth of Mangaba tree (Hancornia speciosa Gomes). Revista Brasileira de Fruticultura, 31(4), 1206–1210.
Esra, K., Işlek, C., & Karayigit, B. (2020). Effects of spermine and putrescine polyamines on capsaicin accumulation in Capsicum annuum L. cell suspension cultures. Acta Agriculturae Slovenica, 115(2), 369–375.
Kalantari, H., Shamsi Ehsan, T., Samimi, A., Kheradmand, P., Shirani, M., & Zeidooni, L. (2018). Histopathological and biomedical parameters determination in the protective effect of hydroalcoholic extract of Allium jesdianum on hepatotoxicity induced by bromobenzene in mice. Iranian Journal of Pharmaceutical Sciences, 14(2), 15–24.
Pirbalouti, A. G. (2019). Phytochemical and bioactivity diversity in the extracts from bulbs and leaves of different populations of Allium jesdianum, a valuable underutilized vegetable. Acta scientiarum Polonorum. Hortorum cultus, 18(2), 115–122.
Dorosti, N., Zarabi, S., Ahmadi, S., Rostami, R., & Rashidi Pour, M. (2017). Anticancer activity evaluation of methanolic extract of Allium Jesdianum and Nectaroscordeum Coelzi against HeLa and K562 cell lines. Yafte, 19(1), 31–41.
Rasool Hassan, B. A. (2012). Medicinal plants (importance and uses). Pharmaceutica Analytica Acta, 3(10), 2153–2435.
Rechinger, K. H. 1963. Flora Iranica. No. 158. Akademische Druke-U, Verlagsanstalt Wien Austria. 49–71.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497.
Golkar, P., Taghizadeh, M., & Yousefian, Z. (2019). The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue and Organ Culture, 137(3), 575–585.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198.
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Miliauskas, G., Venskutonis, P., & Van Beek, T. (2004). Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry, 85(2), 231–237.
Hara, M., Oki, K., Hoshino, K., & Kuboi, T. (2003). Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Science, 164(2), 259–265.
Golkar, P., & Taghizadeh, M. (2018). In vitro evaluation of phenolic and osmolite compounds, ionic content, and antioxidant activity in safflower (Carthamus tinctorius L.) under salinity stress. Plant Cell Tissue Organ Culture, 134(3), 357–368.
Abbas, M. S., El-Shabrawi, H. M., Soliman, A. S., & Selim, M. A. (2018). Optimization of germination, callus induction, and cell suspension culture of African locust beans Parkia biglobosa (Jacq) Benth. Journal of Genetic Engineering and Biotechnology, 16(1), 191–201.
Dewir, Y. H., El-Mahrouk, M. E. S., & Naidoo, Y. (2011). Effects of some mechanical and chemical treatments on seed germination of Sabal palmetto and Thrinax morrisii palms. Australian Journal of Crop Science, 5(3), 248–253.
Elhindi, K. M., Dewir, Y. H., Asrar, A. W., Abdel-Salam, E., El-Din, A. S., & Ali, M. (2016). Improvement of seed germination in three medicinal plant species by plant growth regulators. Horticultural Science, 51(7), 887–891.
Wen-hao, N., & Yan, Z. (2012). Effects of different hormone pretreatments on the germination of Mentha arvensis (peppermint) seeds. Medicinal Plant, 3(11), 64.
Kamenetsky, R., & Gutterman, Y. (2000). Germination strategies of some Allium species of the subgenus Melanocrommuyum from arid zone of Central Asia. Journal of Arid Environments, 45(1), 61–71.
Zdravkovic-Korac, S., Milojevic, J., Tubić, L., Calic-Dragosavac, D., Mitic, N., & Vinterhalter, B. (2010). Somatic embryogenesis and plant regeneration from root sections of Allium schoenoprasum L. Plant Cell Tissue and Organ Culture, 101(2), 237–244.
Monemi, M. B., Kazemitabar, S. K., Khaniki, G. B., Yasari, E., Sohrevardi, F., & Pourbagher, R. (2014). Tissue culture study of the medicinal plant leek (Allium Ampeloprasum L). International Journal of Molecular and Cellular Medicine, 3(2), 118.
Dixit, V., Rai, S. P., & Chaudary, B. R. (2013). Allium sativum: Four-step approach to efficient micropropagation. International Journal of Innovative Biology Research, 2(1), 6–14.
Bekheet, S. A., Taha, H. S., & Solliman, M. E. (2006). Salt tolerance in tissue culture of onion (Allium cepa L.). Arab Journal of Biotechnology, 9(3), 467–476.
Stelmaszczuk, M., & Kozak, D. (2013). Micropropagation of Allium neapolitanum Cirillo. Acta Scientiarum Polonorum Hortorum Cultus, 12(5), 193–206.
Khar, A., Bhutani, R. D., Yadav, N., & Chowdhury, V. K. (2005). Effect of explant and genotype on callus culture and on regeneration in onion (Allium Cepa L.). Akdeniz üniversitesi ziraat fakültesi dergisi., 18(3), 397–404.
Matter, M. A., Hanafy, M. S., & Aly, U. I. (2017). Effect of methyl jasmonate and mannitol application on growth and eugenol content in callus cultures of carnation. Plant Tissue Culture and Biotechnoloy, 27(2), 227–240.
El-Nabarawy, M. A., El-Kafafi, S. H., Hamza, M. A., & Omar, M. A. (2015). The effect of some factors on stimulating the growth and production of active substances in Zingiber officinale callus cultures. Annals of Agricultural Science, 60(1), 1–9.
Abdollahipoor, M., Kalantari, S., Azizi, M., & Saadat, Y. A. (2017). Effects of methyl jasmonate and chitosan on shoot and callus growth of Iranian Hypericum perforatum L. in vitro Cultures. Journal of Medicinal Plants by- Products, 6(2), 165–172.
Bais, H. P., Madhusudhan, R., Bhagyalakshmi, N., Rajasekaran, T., Ramesh, B. S., & Ravishankar, G. A. (2000). Influence of polyamines on growth and formation of secondary metabolites in hairy root cultures of Beta vulgaris and Tagetes patula. Acta Physiologiae Plantarum, 22(2), 151–158.
Das, K., & Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2, 53.
Ali, M. B., Hahn, E. J., & Paek, K. Y. (2007). Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension culture. Molecules, 12(3), 607–621.
Yiu, J. C., Juang, L. D., Fang, D. Y. T., Liu, C. W., & Wu, S. J. (2009). Exogenous putrescine reduces flooding-induced oxidative damage by increasing the antioxidant properties of welsh onion. Scientia Horticulturae, 120(3), 306–314.
Ramirez-Estrada, K., Vidal-Limon, H., Hidalgo, D., Moyano, E., Golenioswki, M., Cusidó, R. M., & Palazon, J. (2016). Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules, 21(2), 182.
Martinez, V. M. V., Estrada-Soto, S. E., de Jesus Arellano-Garcia, J., Rivera-Leyva, J. C., Castillo-Espana, P., Flores, A. F., Cardoso-Taketa, A. T., & Perea-Arango, I. (2017). Methyl jasmonate and salicylic acid enhanced the production of ursolic and oleanolic acid in callus cultures of Lepechinia caulescens. Pharmacognosy Magazine, 13(4), S886.
Alwan, A. H., Twaij, B. M., & Alwan, B. H. (2020). Induction of Allium sativum tissue culture by l-methionine and gibberellic acid and study of the effect of extract against fungal plant pathogens. Plant Archives, 20(1), 2839–2844.
Biondi, S., Antognoni, F., Perellino, N. C., Sacchetti, G., Minghetti, A., & Poli, F. (2004). Medium composition and methyl jasmonate influence the amount and spectrum of secondary metabolites in callus cultures of Zanthoxylum stenophyllum Hemsl. Plant Biosystem, 138(2), 117–124.
Ali, M., Abbasi, B. H., & Ali, G. S. (2015). Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue and Organ Culture, 120(3), 1099–1106.
Saeed, S., Ali, H., Khan, T., Kayani, W., & Khan, M. A. (2017). Impacts of methyl jasmonate and phenyl acetic acid on biomass accumulation and antioxidant potential in adventitious roots of Ajuga bracteosa Wall ex Benth., a high valued endangered medicinal plant. Physiology and Molecular Biology of Plants, 23(1), 229–237.
Al-Maameri, A. A. K., & Almyali, A. A. H. (2020). Effect of putrescine and type of light in callus of Gardenia Jasminoides L. content from some effective medical compounds. In IOP Conference Series: Materials Science and Engineering (Vol. 871, No. 1, p. 012017). IOP Publishing.
Wang, J., Qian, J., Yao, L., & Lu, Y. (2015). Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresources and Bioprocessing, 2(1), 1–9.
Jalalpour, Z., Shabani, L., Afghani, L., Sharifi-Tehrani, M., & Amini, S. A. (2014). Stimulatory effect of methyl jasmonate and squalestatin on phenolic metabolism through induction of LOX activity in cell suspension culture of yew. Turkish Journal of Biology, 38(1), 76–82.
Talukder, P., Talapatra, S., Ghoshal, N., & Sen Raychaudhuri, S. (2016). Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. Journal of the Science of Food and Agriculture, 96(1), 232–244.
Sivanandhan, G., Mariashibu, T. S., Arun, M., Rajesh, M., Kasthurirengan, S., Selvaraj, N., & Ganapathi, A. (2011). The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiologiae Plant, 33(6), 2279.
Taha, L. S., Taie, H. A., & Hussein, M. (2015). Antioxidant properties, secondary metabolites and growth as affected by application of putrescine and moringa leaves extract on jojoba plants. Journal of Applied Pharmaceutical Science, 5(01), 030–036.
Gharib, A. A., & Hanafy Ahmed, A. H. (2005). Response of pea (Pisum sativum, L.) to foliar application of putrescine, glucose, folia feed D and silicon. Journal of Agricultural Science Mansoura University, 30(12), 7563–7579.
Castaneda-Ovando, A., de Lourdes Pacheco-Hernandez, M., Paez-Hernandez, M. E., Rodriguez, J. A., & Galan-Vidal, C. A. (2009). Chemical studies of anthocyanins: A review. Food Chemistry, 113(4), 859–871.
Ram, M., Prasad, K. V., Singh, S. K., Hada, B. S., & Kumar, S. (2013). Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell Tissue and Organ Culture, 113(3), 459–467.
Suan See, K., Bhatt, A., & Lai Keng, C. (2011). Effect of sucrose and methyl jasmonate on biomass and anthocyanin production in cell suspension culture of Melastoma malabathricum (Melastomaceae). Revista de Biologia Tropical, 59(2), 597–606.
Sudha, G., & Ravishankar, G. A. (2003). Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta Physiologiae Plantarum, 25(3), 249–256.
Alrawaiq, N. S., & Abdullah, A. (2014). A review of flavonoid quercetin: Metabolism, bioactivity and antioxidant properties. International Journal PharmTech Research, 6(3), 933–941.
Acknowledgements
The authors would like to thank Research Institute for Biotechnology and Bioengineering, Isfahan University of Technology, Isfahan, Iran, for supporting the practical part of this work. The authors would also thanks from Mostafa Abdollahi Bakhtiari for helping us in experimental sections of elicitation section.
Author information
Authors and Affiliations
Contributions
Esmat Yazdanian done the experimental sections of the study. Dr. Pooran Golkar done the conceptualization, data analysis, material preparation, writing original draft, reviewing, and final editing. Under the supervision of Dr. Mohammad Reza Vahabi, conceptualization and experimental sections were done. Dr. Marzieh Taghizadeh contributed on traits measurement and writing original draft of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
No applicable.
Consent to Participate
No applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yazdanian, E., Golkar, P., Vahabi, M.R. et al. Elicitation Effects on Some Secondary Metabolites and Antioxidant Activity in Callus Cultures of Allium jesdianum Boiss. & Buhse.: Methyl Jasmonate and Putrescine. Appl Biochem Biotechnol 194, 601–619 (2022). https://doi.org/10.1007/s12010-021-03643-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03643-4