Abstract
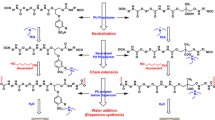
The current study presents an attempt to develop two molecules, namely sulfonated aromatic diol (SAD) and sulfonated aromatic diamine (SADAM), to induce flame retardancy, thermal stability, and dispersion ability for water-based polyurethane dispersions (WPUDs). The previously reported itaconic acid-based polyester polyol was used for prepolymer synthesis as well as for chain extension. Pre- and post-sulfonated compounds were subjected to characterization tests such as FTIR, 13C-NMR, 1H-NMR, and CHNS for confirmation of their structures. The standard dispersant used for the basis of comparison was commercially available dimethyl propionic acid (DMPA). WPUDs were synthesized in different molar concentrations of DMPA, SAD, and SADAM, and the covalent incorporation of all three molecules in the polymer backbone was confirmed by FTIR. The WPUD films were subjected to various thermal tests like TGA and DSC as well as mechanical tests like flexibility and pencil hardness. WPUD films obtained from SADAM showed a remarkable increase in Tg as well as char content. The THRI values for SAD- and SADAM-based films were better than DMPA-based films. SAD- and SADAM-based WPUD films also showed an increase in LOI value and UL-94 ratings with the maximum LOI value of 26. Dispersions based on DMPA showed better stability as compared to dispersions based on SAD and SADAM.








Similar content being viewed by others
References
Namazi, H, “Polymers in Our Daily Life.” BioImpacts, 73–74 (2017). https://doi.org/10.15171/bi.2017.09
Hopewell, J, Dvorak, R, Kosior, E, “Plastics Recycling: Challenges and Opportunities.” Philos. Trans. R Soc. B Biol. Sci., 364 2115–2126 (2009). https://doi.org/10.1098/rstb.2008.0311
Diogo, AC, “Polymers in Building and Construction.” (2015)
Jones, RG, "Polymers for Electronic and Photonic Applications." Polymer (Guildf), 34 3548–3549 (2003)
Barroso, G, Li, Q, Rajendra, K, Bordiab, Motz, G, "Polymeric and Ceramic Silicon-Based Coatings – A Review." J. Mater. Chem. A, 7 1936–1963 (2019). https://doi.org/10.1039/c8ta09054h
Konieczny, J, Loos, K, "Green Polyurethanes from Renewable Isocyanates and Biobased White Dextrins." Polymers (Basel), 11 256 (2019). https://doi.org/10.3390/polym11020256
Akindoyo, JO, Beg, MDH, Ghazali, S, Islam, MR, Jeyaratnam, N, Yuvaraj, AR, “Polyurethane Types, Synthesis, and Applications—A Review.” RSC Adv., 6 114453–114482 (2016). https://doi.org/10.1039/c6ra14525f
Kim, J, Lee, W, Kim, T, Kim, H, Seo, B, Lim, C, Cheong, I, "Synthesis of Thermally Stable Reactive Polyurethane and Its Physical Effects in Epoxy Composites." Appl. Sci., 8 1587 (2018). https://doi.org/10.3390/app8091587
Panwiriyarat, W, Tanrattanakul, V, Pilard, JF, Pasetto, P, Khaokong, C, "Effect of the Diisocyanate Structure and the Molecular Weight of Diols on Bio-based Polyurethanes." J. Appl. Polym. Sci., 130 453–462 (2013). https://doi.org/10.1002/app.39170
Durrieu, V, Gandini, A, "Preparation of Aqueous Anionic Poly(urethane-urea) Dispersions. Influence of the Structure and Molecular Weight of the Macrodiol on the Dispersion and Polymer Properties." Polym. Int., 54 1280–1287 (2005). https://doi.org/10.1002/pi.1844
Gomez, CM, Gutierrez, D, Asensio, M, Costa, V, Nohales, A, "Transparent Thermoplastic Polyurethanes Based on Aliphatic Diisocyanates and Polycarbonate Diol." J. Elastomers Plast., 49 77–95 (2017). https://doi.org/10.1177/0095244316639633
Mahajan, UR, Mhaske, ST, "Kafirin-Derived Films for Sustainable Development by Amidation and Esterification." Polym. Bull., 77 2719–2735 (2019). https://doi.org/10.1007/s00289-019-02876-y
Sheth, P, Mestry, S, Dave, D, Mhaske, S, “Isosorbide-Derived Boron- and Phosphorus-Containing Precursors for Flame-Retardant Epoxy Coating.” J. Coat. Technol. Res., 17 231–241 (2020). https://doi.org/10.1007/s11998-019-00262-x
Rastogi, V, Samyn, P, “Bio-Based Coatings for Paper Applications.” Coatings, 5 887–930 (2015). https://doi.org/10.3390/coatings5040887
Suresh, KI, Kishanprasad, VS, “Synthesis, Structure, and Properties of Novel Polyols from Cardanol and Developed Polyurethanes.” Ind. Eng. Chem. Res., 44 4504–4512 (2005). https://doi.org/10.1021/ie0488750
Mestry, S, Kakatkar, R, Mhaske, ST, “Cardanol Derived P and Si Based Precursors to Develop Flame Retardant PU Coating.” Prog. Org. Coat., 129 59–68 (2019). https://doi.org/10.1016/j.porgcoat.2018.12.016
Li, Y, Luo, X, Hu, S, “Bio-based Polyols and Polyurethanes.” SpringerBriefs in Molecular Science, (2015). https://doi.org/10.1007/978-3-319-21539-6
Patil, DM, Phalak, GA, Mhaske, ST, “Design, and Synthesis of Bio-based UV Curable PU Acrylate Resin from Itaconic Acid for Coating Applications.” Des. Monomers Polym., 20 269–282 (2017). https://doi.org/10.1080/15685551.2016.1231045
Dai, J, Ma, S, Wu, Y, “Polyesters Derived from Itaconic Acid for the Properties and Bio-based Content Enhancement of Soybean Oil-based Thermosets.” Green Chem., 17 2383–2392 (2015). https://doi.org/10.1039/c4gc02057j
Robert, T, Friebel, S, “Itaconic Acid—A Versatile Building Block for Renewable Polyesters with Enhanced Functionality.” Green Chem., 18 2922–2934 (2016). https://doi.org/10.1039/C6GC00605A
Belva, F, Bourbigot, S, Duquesne, S, “Heat and Fire Resistance of Polyurethane-Polydimethylsiloxane Hybrid Material.” Polym. Adv. Technol., 17 304–311 (2006). https://doi.org/10.1002/pat.689
Shi, X, Jiang, S, Zhu, J, Li, G, Peng, X, “Establishment of a Highly Efficient Flame-retardant System for Rigid Polyurethane Foams Based on Bi-phase Flame-retardant Actions.” RSC Adv., 8 9985–9995 (2018). https://doi.org/10.1039/c7ra13315d
Ferriol, M, Cochez, M, Lopez-Cuesta, JM, “Polymers and Fire.” Actual Chim, 41–44 (2008)
Cleetus, C, Thomas, S, Varghese, S, “Synthesis of Petroleum-Based Fuel from Waste Plastics and Performance Analysis in a CI Engine.” J. Energy, 1–10 (2013). https://doi.org/10.1155/2013/608797
Aminabhavi, TM, Cassidy, PE, “Flammability Characteristics of Polymers.” Polym. Plast. Technol. Eng., 28 717–751 (1989). https://doi.org/10.1080/03602558908049824
Prado, G, Jagoda, J, Lahaye, J, “Smoke Formation by Combustion of Polymeric Materials.” Fire Saf. J., 1 229–235 (1978). https://doi.org/10.1016/0379-7112(78)90011-5
Rakotomalala, M, Wagner, S, Doring, M, “Recent Developments in Halogen-Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications.” Materials (Basel), 3 4300–4327 (2010). https://doi.org/10.3390/ma3084300
Tawfik, SY, “Encyclopedia of Polymers and Composites.” Encycl. Polym. Compos., (2014). https://doi.org/10.1007/978-3-642-37179-0_76-2
Jadhav, SD, Jhabarmal, J, “A Review of Non-Halogenated Flame Retardant.” Pharma. Innov. J., 7 380–386 (2018)
Biswas, B, Kandola, BK, “The Effect of Chemically Reactive Type Flame Retardant Additives on the Flammability of PES Toughened Epoxy Resin and Carbon Fiber-Reinforced Composites.” Polym. Adv. Technol., 22 1192–1204 (2011)
Hahladakis, JN, Velis, CA, Weber, R, Iacovissdou, E, Purnell, P, “An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact During Their Use, Disposal, and Recycling.” J. Hazard Mater., 344 179–199 (2018). https://doi.org/10.1016/j.jhazmat.2017.10.014
Yew, MC, Ramli Sulong, NH, Yew, MK, Amalina, MA, Johan, MR, “Influences of Flame-Retardant Fillers on Fire Protection and Mechanical Properties of Intumescent Coatings.” Prog. Org. Coat., 78 59–66 (2015). https://doi.org/10.1016/j.porgcoat.2014.10.006
Georlette, P, Applications of Halogen Flame Retardants. Woodhead Publishing Limited, Cambridge (2011)
Szolnoki, B, Toldy, A, Konrád, P, Szebényi, G, Marosi, G, “Comparison of Additive and Reactive Phosphorus-Based Flame Retardants in Epoxy Resins.” Period Polytech. Chem. Eng., 57 85–91 (2013). https://doi.org/10.3311/PPch.2175
Marosi, G, Szolnoki, B, Bocz, K, Toldy, A, “Reactive and Additive Phosphorus-based Flame Retardants of Reduced Environmental Impact.” Elsevier B.V, (2014). https://doi.org/10.1016/B978-0-444-53808-6.00005-6
Zhao, W, Li, B, Xu, M, Zhang, L, Liu, F, Guan, L, “Synthesis of a Novel Flame Retardant Containing Phosphorus and Sulfur and Its Application in Polycarbonate.” Poly. Eng. Sci, 52 2327–2335 (2012). https://doi.org/10.1002/pen.23192
Chen, Y, Peng, H, Li, J, Xia, Z, Tan, H, “A Novel Flame Retardant Containing Phosphorus, Nitrogen, and Sulfur: Synthesis and Application in Thermoplastic Polyurethane.” J. Therm. Anal. Calorim., 115 1639–1649 (2014). https://doi.org/10.1007/s10973-013-3461-0
Scharte, B, “Phosphorus-Based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development?” Materials (Basel), 3 4710–4745 (2010). https://doi.org/10.3390/ma3104710
Billiani, J, Wilfinger, W, "New Low-VOC Acrylic Polyol Dispersions for Two-Component Polyurethane Coatings.” Surf. Coat. Int. Part B Coat. Int., 85 191–195 (2002)
Tsai, WT, “Toxic Volatile Organic Compounds (VOCs) in the Atmospheric Environment: Regulatory Aspects and Monitoring in Japan and Korea.” Environments, 3 23 (2016). https://doi.org/10.3390/environments3030023
Z, JWW, Table 1. “United States Coatings Shipments.” Wiley Online Libr., 1–74 (2007)
Chen X, Zhang C, Li W, Chen L, Wang W, “Synthesis of Waterborne Polyurethane by the Telechelic α,ω-Di(hydroxy)poly(n-butyl acrylate).” Polymers (Basel), 10 219 (2018). https://doi.org/10.1002/pi.5627
Honarkar, H, “Waterborne Polyurethanes: A Review.” J. Dispers. Sci. Technol., 39 507–516 (2018). https://doi.org/10.1080/01932691.2017.1327818
Wermuth, C, The Practice of Medicinal Chemistry. Elsevier (2008)
Sim, MJ, Cha, SH, “Efficient Polymeric Phosphorus Flame Retardant: Flame Retardancy, Thermal Property, and Physical Property on Polylactide.” Polym. Bull., 76 3463–3479 (2019). https://doi.org/10.1007/s00289-018-2558-9
Jirasutsakul, I, Paosawatyanyong, B, Bhanthumnavin, W, “Aromatic Phosphorodiamidate Curing Agent for Epoxy Resin Coating with Flame-Retarding Properties.” Prog. Org. Coat., 76, 1738–1746 (2013). https://doi.org/10.1016/j.porgcoat.2013.05.009
Chen, Y, Su, Y, Jiao, F, Chen, G, “A Simple and Efficient Synthesis Protocol for Sulfonation of Nitrobenzene Under Solvent-Free Conditions via a Microreactor.” RSC Adv., 2 5637–5644 (2012). https://doi.org/10.1039/c2ra20406a
Mestry, SU, Patil, DM, Mhaske, ST, “Effect of 2-Aminobenzothiazole on Antimicrobial Activity of Waterborne Polyurethane Dispersions (WPUDs).” Polym. Bull., 76 1899–1914 (2019). https://doi.org/10.1007/s00289-018-2469-9
Born, L, Hespe, H, “On the Physical Crosslinking of Amine-Extended Polyurethane Urea Elastomers: A Crystallographic Analysis of Bis-urea from Diphenyl Methane-4-Isocyanate and 1,4-Butane Diamine.” Colloid Polym. Sci., 263 335–341 (1985). https://doi.org/10.1007/BF01412250
Baudonnet, L, Pere, D, Michaud, P, Grossiord, JL, Rodriguez, F, “Effect of Dispersion Stirring Speed on the Particle Size Distribution and Rheological Properties of Carbomer Dispersions and Gels.” J. Dispers. Sci. Technol., 23(4) 499–510 (2002). https://doi.org/10.1081/DIS-120014018
Wei, L, Yue, G, Hongjian, P, Hongying, C, “Dispersion Stability of Titanium Dioxide in Aqueous Isopropanol with Polymer Dispersant.” J. Coat. Technol. Res., 17 (4) 1083–1090 (2020). https://doi.org/10.1007/s11998-020-00323-6
Forney, MW, Poler, JC, “Significantly Enhanced SWCNT Dispersion Stability in Mixed Solvent Systems.” Langmuir, 1–3 (2011). https://doi.org/10.1021/jp202559m.
Rusinska-Roszak, D, Sowinski, G, “Estimation of the Intramolecular O-H⋯O=C Hydrogen Bond Energy via the Molecular Tailoring Approach. Part I: Aliphatic Structures.” J. Chem. Inf. Model., 54 (7) 1963–1977 (2014). https://doi.org/10.1021/ci500107w
Pesetskii, SS, Jurkowski, B, Krivoguz, YM, Kelar, K, “Free-Radical Grafting of Itaconic Acid Onto LDPE by Reactive Extrusion: I. Effect of Initiator Solubility.” Polymer (Guildf), 42 (2) 469–475 (2001). https://doi.org/10.1016/S0032-3861(00)00356-6
Psimadas, D, Georgoulias, P, Valotassiou, V, Loudos, G, “Molecular Nanomedicine Towards Cancer.” J. Pharm. Sci. 101 (7) 2271–2280 (2012). https://doi.org/10.1002/jps
Balani, K, Verma, V, Agarwal, A, Narayan, R, “Physical, Thermal, and Mechanical Properties of Polymers.” Biosurfaces, 329–344 (2015). https://doi.org/10.1002/9781118950623.app1
Mehdipour-Ataei, S, Tabatabaei-Yazdi, Z, “Heat Resistant Polymers.” Encycl. Polym. Sci. Technol., 1–31 (2015). https://doi.org/10.1002/0471440264.pst636
Rogulska, M, Kultys, A, Puszka, A, “New Thermoplastic Poly(carbonate-urethane)s Based on Chain Extenders with Sulfur Atoms.” Chem. Pap., 71 1195–1204 (2017). https://doi.org/10.1007/s11696-016-0112-5
Teo, L, Chen, C, Kuo, J, “Fourier Transform Infrared Spectroscopy Study on Effects of Temperature on Hydrogen Bonding in Amine-Containing Polyurethanes and Poly(urethane-urea)s.” Macromolecules, 30 1793–1799 (1997). https://doi.org/10.1021/ma961035f
Yang, X, Guo, Y, Luo, X, “Self-healing, Recoverable Epoxy Elastomers and Their Composites with Desirable Thermal Conductivities by Incorporating BN Fillers via In-situ Polymerization.” Compos. Sci. Technol., 164 59–64 (2018). https://doi.org/10.1016/j.compscitech.2018.05.038
Patel, M, Shukla, J, Patel, N, Patel, K, “Biomaterial Based Novel Polyurethane Adhesives for Wood to Wood and Metal to Metal Bonding.” Mater. Res., 12 385–393 (2009). https://doi.org/10.1590/S1516-14392009000400003
Panico, M, Narayanan, S, Brinson, LC, “Simulations of Tensile Failure in Glassy Polymers: Effect of Cross-Link Density.” Model Simul. Mater. Sci. Eng., 18 (2010). https://doi.org/10.1088/0965-0393/18/5/05500
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dave, D., Mestry, S. & Mhaske, S.T. Development of flame-retardant waterborne polyurethane dispersions (WPUDs) from sulfonated phosphorus-based reactive water-dispersible agents. J Coat Technol Res 18, 1037–1049 (2021). https://doi.org/10.1007/s11998-020-00458-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-020-00458-6