Abstract
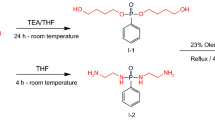
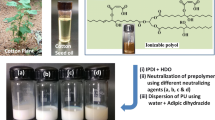
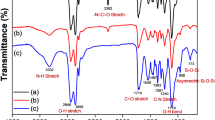
The objective of the current research work is to prepare a difunctional reactive dispersing agent derived from cardanol which can be used as an alternative for dimethylol propionic acid (DMPA) in waterborne polyurethane dispersion synthesis. The novelty of the research lies between the use of bio-based resource and utilizing the sulfonic acid group as an anionic dispersible group. The cardanol was sulfonated using oleum followed by the reaction of the hydroxyl group of phenol with the epichlorohydrin (ECH). The obtained product was then hydrolyzed to generate double functionalities which can be introduced in the PU backbone through a chemical reaction with a diisocyanate. The obtained intermediates and product were characterized using hydroxyl values, epoxy equivalent weights (EEW), CHNS analysis, FTIR and 1H-NMR analysis. The typical acetone process was used for the preparation of WPUDs, and the cured films were further analyzed for the various coating properties in which thermal properties showed significant improvements with the incorporation of HESC to the PU system along with the increased char yield and glass transition temperature (Tg), whereas the mechanical properties did not show any improvements, which could be attributed to the bulky structure of HESC and increased rigidity in the polymeric network. The detailed synthesis, characterizations and obtained results are presented and discussed here.
Graphic abstract










Similar content being viewed by others
Change history
21 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s00289-020-03504-w
References
Chattopadhyay DK, Raju KVSN (2007) Structural engineering of polyurethane coatings for high performance applications. Prog Polym Sci 32:352–418. https://doi.org/10.1016/j.progpolymsci.2006.05.003
Nečasová B, Liška P, Kelar J, Šlanhof J (2019) Comparison of adhesive properties of polyurethane adhesive system and wood-plastic composites with different polymers after mechanical, chemical and physical surface treatment. Polymers (Basel) 11:15–17. https://doi.org/10.3390/polym11030397
Segura DM, Nurse AD, McCourt A et al (2005) Chemistry of polyurethane adhesives and sealants. Handb Adhes Sealants 1:101–162. https://doi.org/10.1016/S1874-5695(02)80004-5
Akindoyo JO, Beg MDH, Ghazali S et al (2016) Polyurethane types, synthesis and applications-a review. RSC Adv 6:114453–114482. https://doi.org/10.1039/c6ra14525f
Paik Sung CS, Schneider NS (1978) Structure-property relationships of polyurethanes based on toluene di-isocyanate. J Mater Sci 13:1689–1699. https://doi.org/10.1007/BF00548732
Berezkin Y, Urick M (2013) Modern polyurethanes: Overview of structure property relationship. ACS Symp Ser 1148:65–81. https://doi.org/10.1021/bk-2013-1148.ch004
Honarkar H (2018) Waterborne polyurethanes: a review. J Dispers Sci Technol 39:507–516. https://doi.org/10.1080/01932691.2017.1327818
Overbeek GC, Steenwinkel P, Tennebroek R, Nabuurs T (2003) US Patent 20030055171A1
Costa G, Kroutilova IA, Mercatali S, Bassi GL (2013) EP Patent 2321361B1
Duan Y, Dochniak MJ, Stammler S (1994) WO Patent 1995008583A1
Wei X, Yu X (1997) Synthesis and properties of sulfonated polyurethane ionomers with anions in the polyether soft segments. J Polym Sci Part B Polym Phys 35:225–232. https://doi.org/10.1002/(SICI)1099-0488(19970130)35:2%3c225::AID-POLB3%3e3.0.CO;2-R
Yang Z, Zang H, Wu G (2019) Study of solvent-free sulfonated waterborne polyurethane as an advanced leather finishing material. J Polym Res 26:1–13. https://doi.org/10.1007/s10965-019-1884-4
Feng J, Wang X, Guo P et al (2018) Mechanical properties and wear resistance of sulfonated graphene/waterborne polyurethane composites prepared by in situ method. Polymers (Basel) 10:1–12. https://doi.org/10.3390/polym10010075
Deka R, Bora MM, Upadhyaya M, Kakati DK (2015) Conductive composites from polyaniline and polyurethane sulphonate anionomer. J Appl Polym Sci 132:1–9. https://doi.org/10.1002/app.41600
Bottino A, Capannelli G, Comite A, Costa C (2011) Synthesis and characterization of polyurethanic proton exchange membranes. J Fuel Cell Sci Technol 8:2–8. https://doi.org/10.1115/1.4003981
Mannari VM (2015) US Patent 8,952,093
Fu C, Zheng Z, Yang Z et al (2014) A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material. Prog Org Coatings 77:53–60. https://doi.org/10.1016/j.porgcoat.2013.08.002
Liang H, Feng Y, Lu J et al (2018) Bio-based cationic waterborne polyurethanes dispersions prepared from different vegetable oils. Ind Crops Prod 122:448–455. https://doi.org/10.1016/j.indcrop.2018.06.006
Panda SS, Panda BP, Nayak SK, Mohanty S (2018) A review on waterborne thermosetting polyurethane coatings based on castor oil: synthesis, characterization, and application. Polym - Plast Technol Eng 57:500–522. https://doi.org/10.1080/03602559.2016.1275681
Gedam PH, Sampathkumaran PS (1986) Cashew nut shell liquid: extraction, chemistry and applications. Prog Org Coat 14:115–157. https://doi.org/10.1016/0033-0655(86)80009-7
Kumar PP, Paramashivappa R, Vithayathil PJ, Rao PVS, Rao AS (2002) Process for Isolation of Cardanol from Technical Cashew (Anacardium occidentale L. ) Nut Shell Liquid. J Agric Food Chem 50(16):4705–4708. https://doi.org/10.1021/jf020224w
Shi Y, Kamer PCJ, Cole-Hamilton DJ (2019) Synthesis of pharmaceutical drugs from cardanol derived from cashew nut shell liquid. Green Chem 21:1043–1053. https://doi.org/10.1039/c8gc03823f
Chen J, Liu Z, Jiang J et al (2015) A novel biobased plasticizer of epoxidized cardanol glycidyl ether: synthesis and application in soft poly(vinyl chloride) films. RSC Adv 5:56171–56180. https://doi.org/10.1039/c5ra07096a
Balachandran VS, Jadhav SR, Vemula PK, John G (2013) Recent advances in cardanol chemistry in a nutshell: from a nut to nanomaterials. Chem Soc Rev 42:427–438. https://doi.org/10.1039/c2cs35344j
Mele G, Vasapollo G (2008) Fine chemicals and new hybrid materials from cardanol. Mini Rev Org Chem 5:243–253. https://doi.org/10.2174/157019308785161611
Lubi MC, Thachil ET (2000) Cashew nut shell liquid (CNSL)–A versatile monomer for polymer synthesis. Des Monomers Polym 3:123–153. https://doi.org/10.1163/156855500300142834
Bruce IE, Mehta L, Porter MJ et al (2009) Anionic surfactants synthesised from replenishable phenolic lipids. J Surfactants Deterg 12:337–344. https://doi.org/10.1007/s11743-009-1116-8
Peungjitton P, Sangvanich P, Pornpakakul S et al (2009) Sodium cardanol sulfonate surfactant from cashew nut shell liquid. J Surfactants Deterg 12:85–89. https://doi.org/10.1007/s11743-008-1082-6
Mestry SU, Patil DM, Mhaske ST (2018) Effect of 2-aminobenzothiazole on antimicrobial activity of waterborne polyurethane dispersions (WPUDs). Polym Bull. https://doi.org/10.1007/s00289-018-2469-9
Dogan NA (2014) Thesis: Synthesis and Characterization of novel waterborne polyurethane dispersions. Sabanci University, Graduate School of Engineering and Natural Science
Honarkar H, Barmar M, Barikani M (2015a) New sulfonated waterborne polyurethane dispersions: preparation and characterization. J Dispers Sci Technol 2691:151006122122007. https://doi.org/10.1080/01932691.2015.1028071
Honarkar H, Barmar M, Barikani M (2015b) Synthesis, characterization and properties of waterborne polyurethanes based on two different ionic centers. Fibers Polym 16:718–725. https://doi.org/10.1007/s12221-015-0718-1
Anderson JT (2003) US Patent 6,649,727 B1
Gao R, Zhang M, Dixit N, Moore R, Long T (2012) Influence of ionic charge placement on performance of poly(ethylene glycol)-based sulfonated polyurethanes. Polymer 53:1203–1211. https://doi.org/10.1016/j.polymer.2012.01.043
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised to correct the corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mestry, S.U., Khuntia, S.P. & Mhaske, S.T. Development of waterborne polyurethane dispersions (WPUDs) from novel cardanol-based reactive dispersing agent. Polym. Bull. 78, 6819–6834 (2021). https://doi.org/10.1007/s00289-020-03450-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03450-7