Abstract
Egg pasta contains high amount of cholesterol, that upon oxidation, generates oxysterols (COPs), which play a key role in the onset of several human diseases. In this study, the effect of two tannins (esters of ellagic acid, A; esters of gallic acid, B) at three different concentrations (0.25%, 0.50%, 1.00%) was tested in egg pasta considering two different pasta shapes (squared, S; rectangular, F). When tannin B was added, the total phenolic content (TPC) in fresh pasta increased (p < 0.01) and after cooking its content was greater than those obtained with tannin A. The pasta shape affected the presence of cholesterol; its amount in uncooked F shape samples (27.67 ± 0.28 mg/g pasta) was higher than that found in S shape (21.18 ± 0.49 mg/g pasta). In addition, tannin B significantly (p < 0.01) increased the presence of cholesterol in the cooking water (up to 1.04 ± 0.05 μg/mL), in particular in S pasta shape. Tannin B was also greater than tannin A to reduce the content of COPs in fresh egg pasta, while the cooking process did not impact (p > 0.05) the oxidation of cholesterol. The results suggest that tannin B could be applied in the formulation of egg pasta as a strategy for reducing the content of cholesterol and its oxidation products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egg pasta represents a typical Italian food product rich in eggs (at least four hens’ eggs or 200 g of hens’ eggs per kilogram of semolina (Presidential Decree, 2001)) and with a higher cholesterol content than traditional pasta. However, it might be highlighted that cholesterol, due to different reactions occurring during the processing and storage, in a similar way to monounsaturated fatty acids, may be oxidized generating cholesterol oxidation products (oxysterols, COPs), which negatively impact human health (Ansorena et al., 2013). COPs play a key role in the development of atherosclerotic plaques and in the onset of major chronic inflammatory processes (Cardenia et al., 2017; Gooding & de Ferranti, 2010; Mortensen & Nordestgaard, 2020; Nelson, 2013). Moreover, Malaguti et al. (2019) explained the role of COPs in altering the brain cholesterol homeostasis underlining their role in different neurodegenerative diseases (e.g., Alzheimer’s, Parkinson’s, and Huntington’s diseases). Others (de Oliveira et al., 2018) reported the potential mutagenic and genotoxic effects of oxysterols. Thus, the correlation between COPs and human pathophysiology has been receiving increased attention (Kloudova et al., 2017). On the other hand, the COPs’ functional groups including hydroxyl, hydroperoxyl, epoxy, and keto, increase the intestinal absorption of COPs more than cholesterol impacting the cholesterol/lipid metabolism and membrane’s function (Poli et al., 2022).
Thus, the COPs were largely determined in egg products as related to processing and storage conditions. As reported by Chudy and Teichert (2021) eggs and egg-derived products represent the main dietary sources of COPs. The pasta processing consists of steps such as mixing, extrusion, drying and also cooking that can promote the initiation of oxidative reactions, caused by high temperature, presence of metals, oxygen, and exposure to light in presence of photosensitizers (photooxidation), which lead to high production of COPs. For instance, Verardo et al. (2017) described a drastic increase in COPs content when a high temperature was applied to drying egg pasta. Again, food products formulated with pasteurized eggs displayed higher amount of COPs than those with spray-dried eggs (Verardo et al., 2020).
It is also well known that pasta shape is strictly related to macroscopic structural attributes, which could reflect different oxidative and retention behavior. As reported by literature, the pasta shape affects water absorption and solid loss; for instance, the penne shape absorbs less water than spaghetti, while both spaghetti and penne shapes lose fewer solids in cooking water than the risoni shape (Suo et al., 2021). The long pasta shape showed a higher glycaemic index than that of the short pasta shape (Pugnaloni et al., 2021); again, pasta shape together with gluten affects the texture, mastication behavior, and retention of compounds as related to protein continuous network with embedded gelatinized starch (Suo et al., 2021).
To date, the interest in the development of novel or fortified food products with antioxidants drastically increased (de Oliveira et al., 2018; Sharma et al., 2022). Several natural compounds obtained from vegetal sources or their processing by-products may represent interesting and promising alternatives to replace synthetic ones (Barbieri et al., 2021; Espinosa et al., 2015; Dutta et al., 2022; Sabaghi et al., 2022). The maqui berry powder was tested in fresh pasta for increasing the polyphenols content and antioxidant capacity and to reduce the glycemic index (Bianchi et al., 2022). An increased amount of phenol compounds, a boosted antioxidant activity, and reduced digestible starch was also found in pasta fortified with olive pomace (Simonato et al., 2019). Similar results in terms of enhanced antioxidant properties were also found when mango peel dietary fiber concentrate was added at 5% in macaroni pasta (Garcia-Amezquita et al., 2018). Moreover, antioxidant properties of parboiled wheat noodles were improved by fortification with pomegranate peel extract (Kazemi et al., 2017).
Tannins have been receiving rising interest (Al-Hijazeen et al., 2016; Fruet et al., 2020; Gülçin et al., 2010). The tannins, water-soluble polyphenols, are a heterogeneous group of high molecular weight, plant bioactive compounds, that can be found in fruits, vegetables (in particular leaves, peels or seeds), certain grains, cocoa/chocolate, and beverages such as coffee, tea, and wine (Fraga-Corral et al., 2021; Lamy et al., 2016). They contribute to exert positive health effects due to their anti-inflammatory, anti-diabetic, cardioprotective, healing, and antimicrobial (antiviral and antibacterial) activity since they may act as antioxidants, scavenging free radicals and inhibiting lipid peroxidation and gut microbiota modulating (Fraga-Corral et al., 2021; Lamy et al., 2016; Smeriglio et al., 2017). Recently, tannins were also investigated as wall material in microencapsulated sacha inchi oil (da Silva Soares et al., 2021), Origanum onites L. essential oil (Karagozlu et al., 2021) and as natural preservative in cooked chicken meat (Al-Hijazeen et al., 2016). Moreover, tannins are extensively investigated since they bind biological macromolecules such as proteins, polysaccharides, and in particular lipids and cholesterol impacting liposomes and lipid vesicles fluidity (Lamy et al., 2016). As reported by Sewoski et al. (2022), tannins interact with lipids in a similar manner and magnitude observed for proteins revealing a possible competition between lipids and proteins. On the other hand, Zeng et al. (2020) explained that apple condensed tannins directly interact with cholesterol leading to coprecipitation and cholesterol lowering effect.
Hence, these beneficial properties together with consolidated extraction techniques from natural sources make tannins promising compounds to be integrated into egg pasta formulation for increasing the content of the bioactive compounds, reducing cholesterol and oxysterols amounts.
Thus, the aim of the present study was to evaluate the ability of two different types of condensed tannins to impact the retention of cholesterol and its oxidation products in fresh and cooked egg pasta as related to two different pasta shapes.
Materials and Methods
Materials
All chemicals and solvents were of analytical grade. Millipore membrane filters (0.45 μm and 0.20 μm), chloroform, n-hexane, methanol, water (≥ 99.8%), and ethanol were purchased from Merck (Darmstadt, Germany). N° 1 filters (70-mm diameter) were provided from Whatman (Maidstone, England). The standard mixtures of free fatty acids (GLC 469) were purchased from Nu-Chek Prep, Inc. (Elysian, MN, USA). Folin–Ciocalteu’s phenol reagent, triolein (≥ 99.0%), tripalmitin (≥ 99.0%), tristearin (≥ 99.0%), 1,3-diolein (≥ 99.0%), 1,2(3)-dipalmitin (≥ 99.0%), cholesteryl palmitate (≥ 97%), 1-oleoyl-rac-glycerol (≥ 99.0%), methyl tridecanoate (≥ 99.5%), 5-cholesten-3β-ol (cholesterol, ≥ 99%), 5α-cholestane (≥ 97%), anhydrous pyridine (99.8%), and N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane (BSTFA:TMCS, 99:1, v/v) were purchased from Sigma (St. Louis, MO, USA). 2–2′-Diphenyl-1-picrylhydrazyl (DPPH•), gallic acid (≥ 98%) were provided by Fluka (Milan, Italy). Sodium carbonate and potassium hydroxide were purchased from Carlo Erba (Milan, Italy). Tannins with ellagic acid esters of glucose, from oak wood extract (A), and gallic acid esters of quinic acid, from purified and deodorized tara pods extract (B), were kindly provided by a local company.
Pasta Sampling
The pasta dough was prepared by mixing and kneading 160 g of wheat flour (type 00), 80 g of durum wheat semolina flour, 100 g of pasteurized egg product, tannins, and 40 mL of water in a mixer bowl (Dolly, Imperia & Monferrina S.p.A., Moncalieri, Italy) until a compact and homogeneous mixture was obtained. Tannins (powder) were added in different percentages of the total flour amount namely 0.25% (w/w), 0.50% (w/w), and 1.00% (w/w). Afterward, two different pasta shapes, squared shape (S) and rectangular shape (F), were obtained from the same dough by using a bronze die-extruder in order to produce pasta characterized by the same volume but a different shape (Fig. 1). Control without the addition of the tannins was also prepared for both S and F shapes. The pasta samples obtained were left to air dry on traditional hangers. Lastly, the samples were cooked in ultrapure water at 1:10 pasta/water ratio (w/w) (Cappa & Alamprese, 2017) for 4 min until the white center core of the pasta disappeared. Three independent experiments (n = 3) were conducted. All pasta samples were freeze-dried (Lio 5P, 5 Pascal, Italy) and ground immediately before being analyzed.
Extraction of Antioxidant Compounds
The extraction of the antioxidant compounds from both uncooked and cooked pasta was performed according to Fares et al. (2010) with a modified weight/solvent ratio (1:20, w/v). In brief, 20 mL of a methanol:water solution (80:20; v/v) acidified with formic acid (to reach a pH equal to 2.5) was added to 1 g of ground pasta and stirred in darkness for 2 h. Subsequently, the sample was centrifuged (12,900 × g, 15 min, 5 °C) and the supernatant was collected. The extraction was then repeated two times and the supernatants were pooled, filtered through a Millipore filter (PTFE; 0.45 μm) and stored in amber glass vials at 4 °C until subsequent analyses.
Total Phenolic Content
The determination of the total phenolic content (TPC) was carried out by the Folin–Ciocalteu colorimetric method described by Singleton and Rossi (1965) with slight modifications as reported by Cantele et al. (2020), for adapting it to a BioTek Synergy HT spectrophotometric multi-detection 96-well microplate reader (BioTek Instruments, Milan, Italy). The analysis was run in triplicate. Results were expressed as milligrams of gallic acid equivalents (GAE) per gram of dry pasta.
Radical Scavenging Activity
The radical scavenging activity (RSA) was determined through the inhibition of the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) free radical according to the method described by Gadow et al. (1997) with some modifications to adapt it to a BioTek Synergy HT microplate reader (Barbosa-Pereira et al., 2018). Results were expressed as μmol of Trolox equivalents (TE) per gram of dry pasta.
Determination of Total Cholesterol
The total cholesterol was extracted according to Kuczyńska et al. (2019) with some modifications. Briefly, 3 mL of KOH (4 M) in methanol was added to 100 mg of ground lyophilized sample, together with 1 mg of 5α-cholestane (internal standard 1; IS1) and 0.5 mg of 19-hydroxycholesterol (internal standard 2; IS2), used as internal standards to quantify cholesterol and cholesterol oxidation products, respectively. Samples were stirred in darkness for 18 h at room temperature (25 °C) and then 10 mL of chloroform and 10 mL of a citric acid solution (0.1%; w/v) were added. After centrifugation (3600 × g for 15 min at 10 °C), the organic phase (subnatant), containing the cholesterol, was collected. The extraction was then repeated, and the two organic phases were combined. The solvent was removed by flushing nitrogen and the unsaponifiable fraction solubilized with an n-hexane:isopropanol solution (3:2; v/v) and stored at −18 °C. Once extracted, the cholesterol-containing fraction was subjected to silylation in order to obtain the trimethylsilyl ethers (TMS). In detail, 200 μL of the unsaponifiable matter was dried under nitrogen flow; then, 200 μL of pyridine and 180 μL of BSTFA (with 1% TMCS) were added; the reaction was carried out for 30 min at 60 °C under stirring. Lastly, the solvent was evaporated and TMS derivatives were dissolved in 100 μL of n-hexane. One microliter was injected into a Shimadzu QP2010 Plus GC/MS (Shimadzu, Kyoto, Japan) equipped with a fused silica capillary column (RXi-5 ms, 10 m × 0.1 mm i.d. × 0.1 μm film thickness; Restek, Bellefonte, PA). The oven temperature ramped from 220 to 330 °C (at 10 °C/min) and then maintained at 350 °C for 2 min. The temperature of injector and the interface were set at 325 °C and 330 °C, respectively. The injection was performed in split mode (1:30 ratio) using an autosampler AOC-5000 Pal (Shimadzu, Kyoto, Japan). Helium was chosen as carrier gas with a linear velocity of 47.0 cm/s. Acquisitions and peak integrations were performed in TIC (total ion current) and SIM (single ion monitoring), respectively.
Determination of Lipids in Cooking Water
After cooking pasta, the water was collected, weighed, and transferred into a separating funnel. Afterwards, the lipid extraction was performed as suggested by Folch et al. (1957). The extraction was performed twice, and the organic phases were pooled and filtered through a Whatman filter paper to remove any solid suspensions. The solvent was then evaporated and the lipid matter was dissolved in 200 μL of n-hexane:isopropanol (3:2; v/v) containing 10 μg of 5α-cholestane (internal standard, IS). According to Luise et al. (2018), 1 μL of sample was injected in a GC-FID system (GC-2010 Plus, Shimadzu, Kyoto, Japan) equipped with a Rtx®-5 fused silica capillary column (20 m × 0.10 mm i.d. × thickness 0.10 μm; Restek, Bellefonte, PA) in split mode (1:50 ratio). The oven temperature was programmed from 100 to 350 °C at a rate of 5 °C/min. The final temperature was maintained for 20 min. The injector and FID temperature was set at 348 °C and 350 °C, respectively. Helium was chosen as the carrier gas with a linear velocity of 47.0 cm/s. The free fatty acids, free and esterified cholesterol, monoacylglycerols, diacylglycerols, and triacylglycerols were recognized by injection of pure commercial standards under the same analytical conditions.
Determination of Cholesterol Oxidation Products
Cholesterol oxidized products (COPs) were isolated from the unsaponifiable matter according to Rose-Sallin et al. (1995). Briefly, an SPE-NH2 cartridge, containing 3 mm of anhydrous sodium sulfate, was activated with 3 mL of n-hexane. Then, the 9/10 of the unsaponifiable fraction was loaded and eluted with 6 mL of n-hexane:ethylacetate (95:5; v/v) and 10 mL of n-hexane:ethylacetate (9:1; v/v). Once purified, COPs were eluted through the column with 10 mL of acetone and collected. Subsequently, the acetone was removed under nitrogen flow and the COPs were derivatized as described in the “Determination of Total Cholesterol” section. Samples were dissolved in 100 μL of n-hexane and injected into a Shimadzu QP2010 Plus GC/MS (Shimadzu, Kyoto, Japan) equipped with a RXi-5 ms fused silica capillary column (10 m × 0.10 mm i.d. × thickness 0.10 μm; Restek, Bellefonte, PA, USA) as reported by Cardenia et al. (2012). The injection (1 μL) was performed in splitless mode. The temperature was programmed from 250 to 325 °C at 20 °C/min. The injector temperature was set at 325 °C and the ion source temperature was set at 200 °C. Helium was used as the carrier gas with a linear velocity of 43.0 cm/s. The COPs were recognized by their mass spectra (TIC) and quantified by single ion monitoring (SIM). The quantifier ion and the three qualifier ions are shown in Supplementary Table S1 (Supplementary material section). The acquisition and integration modes were in TIC (total ion current) and SIM (single ion monitoring), respectively, using the qualifier and quantifier ions.
The oxidation factor was calculated according to the following equation:
where cholesterol represents the cholesterol content in pasta sample and COPs the cholesterol oxidation products content.
Statistical Analysis
Results were reported as means and standard deviation of three independent replicates and were statistically processed using SPSS Statistics software (version 25.0; IBM, Chicago, USA). Analysis of variance (ANOVA) and Tukey’s post hoc test, with 95% confidence level, were used to identify significant differences between the mean values of the groups as related to the different tannins, their concentrations, the pasta shapes, and the cooking effect. To further understand the data variability, principal component analysis (PCA) was carried out.
Results and Discussions
Total Phenolic Content
The effect of two tannins on the total phenolic content (TPC) in cooked pasta as related to different shapes is depicted in Fig. 2. In uncooked pasta, the pasta without tannins (control) exhibited a TPC equal to 1.21 ± 0.00 mg GAE/g and the addition of tannins in the formulation increased the TPC. These results are in agree with those reported by Bianchi et al. (2022). The S pasta shape fortified with tannin A showed a TPC ranged from 1.56 to 2.46 mg GAE/g, while F shape displayed a TPC ranged between 1.55 and 2.49 mg GAE/g. In S pasta shape, the presence of tannin A at both 0.5 and 1.0% led to the highest value of TPC, while in F shape only with 1.0% of tannin A the greatest value was reached. The tannin B displayed greater TPC than tannin A; in fact, the content of phenols increased at least twice; the TPC ranged between 2.76 and 6.31 mg GAE/g in S pasta shape and in a similar way ranged from 2.73 to 6.10 mg GAE/g in F pasta shape. However, it might be pointed out that no significant differences (p > 0.05) due to the different shapes were observed but a significant effect due to the type and concentration of tannin was detected. In order to evaluate the possible losses of the bioactive compounds due to the cooking process, the TPC was also evaluated in cooked samples (Table 1). In agree with literature (Lisiecka et al., 2019), the cooking process significantly decreased the TPC (Fig. 2). The TPC loss was more marked when tannin A was used; both F and S shape pasta with 1% of tannin A shown the highest loss (by 41% and 42% of TPC, respectively). On the other hand, pasta with tannin B lost less phenolic compounds since the loss was less than 15% (at 1.0% of tannin concentration). It might be highlighted that TPC decrease in cooked samples could be ascribed to the high temperatures when exposed to boiling water and to their solubilization in the cooking water as reported by literature (Alide et al., 2020; Gunathilake et al., 2018; Simonato et al., 2019). Furthermore, as reported by Tanaka et al. (2018) the solubility of tannins in water is additionally influenced by their molecular structure and flexibility. However, the TPC found in the samples containing tannin B at each concentration and with tannin A at 0.5 and 1% was higher than that observed by Tolve et al. (2020), where pasta fortified with 5% grape pomace powder displayed a TPC equal to 1.28 and 1.05 mg/g GAE before and after cooking, respectively. Moreover, lower values were also found by Simonato et al. (2019) when olive pomace powder was integrated at 5% in pasta, obtaining a TPC of 1.29 and 0.38 mg/g GAE for uncooked and cooked pasta, respectively.
Total phenolic content (TPC) (mg GAE/g dry pasta) of pasta added with tannins before (filled bars) and after (dot-patterned bars) cooking in (a) squared shape (S); (b) rectangular shape (F) for both tannins (A and B). Each bar represents the mean ± standard deviation of three independent experiments (n = 3). Each sample is named with the combination of two letters indicating the shape (S; F) and added tannin (A; B). Results were analyzed trough Tukey’s post hoc test. Significance: *p < 0.05; **p < 0.01; ***p < 0.001
Radical Scavenging Activity (RSA)
The antiradical activity determined in both uncooked and cooked pasta with and without tannins for both S and F shapes is reported in Fig. 3. In general, as the concentration of tannins increased, the RSA significantly raised; however, no significant differences (p > 0.05) were reported due to different shapes. It might be pointed out that the two tannins displayed a different behavior since pasta with tannin A exhibited a lower RSA than pasta with tannin B at the three levels of considered concentrations in both uncooked and cooked samples. Thus, the gallic acid ester tannin was confirmed to be the best performer in egg pasta, with a substantial increase in both TPC and RSA values with no significant differences due to the different pasta shapes considered. The great gap observed between the two tannins can be again ascribed to their different chemical structure and also reflects the results obtained for TPC. In fact, the ability of phenolic compounds to exert antioxidant activities, including scavenging free radicals, is mainly based on the number of hydroxyl groups and their position on the aromatic ring characteristic of polyphenols (Kumar & Goel, 2019). In addition, the resulting activity could also be related to the susceptibility of bioactive compounds to interact with macromolecules present in the dough (Sęczyk et al., 2021). However, the ability of the functional groups present in the chemical structure of tannins provides them with the ability to create stable bonds with other molecules, such as proteins or carbohydrates, reducing their antioxidant activity (Fraga-Corral et al., 2020; Minatel et al., 2017).
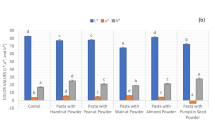
Radical scavenging activity (RSA) (μmol TE/g dry pasta) of pasta added with tannins before (filled bars) and after (dot-patterned bars) cooking in (a) squared shape (S); (b) rectangular shape (F) for both tannins. Each bar represents the mean ± standard deviation of three independent experiments (n = 3). Results were analyzed trough Tukey’s post hoc test. Significance: *p < 0.05; **p < 0.01; ***p < 0.001. Each sample is named with the combination of two letters indicating the shape (S; F) and added tannin (A; B)
After cooking (Fig. 3), in pasta fortified with 0.25% of tannin A (F shape) and with 1.00% of tannin B (S shape), the RSA increased from 3.55 ± 0.14 μmol TE/g to 6.47 ± 0.14 μmol TE/g and from 81.27 ± 1.03 μmol TE/g to 96.29 ± 3.11 μmol TE/g (by 18.5% increase), respectively. These results could be ascribed to changes in the structure of tannins and the food matrix due to heat treatment. During the cooking process, tannins can be degraded by high temperature and/or hydrolyzed with a consequent leaching of free forms (Karim et al., 2017; Moreno et al., 2018; Parada & Aguilera, 2007). These results are consistent with those found in pasta fortified with maqui (Bianchi et al., 2022) or with olive pomace (Simonato et al., 2019).
Cholesterol Content in Pasta
It is well known that egg pasta contains a high amount of cholesterol and the processing as well as ingredients could affect its content in significant way (Verardo et al., 2017). On the other hand, different strategies were tested for developing egg products with reduced amount of cholesterol (Lamas et al., 2016). Since natural extracts may contain lipid compounds, the quality control of tannins was assessed by extraction of potential lipid matter and analysis by GC-FID. The results confirmed that no lipids were in powder tannins (data not shown).
In general, the pasta shape affected the presence of cholesterol since its content in uncooked pasta without tannins (control) was significantly (p < 0.05) higher in the F pasta shape. In agree with the literature, the larger surface area of the F shape could contribute to a higher moisture transfer by diffusion (Srikiatden & Roberts, 2007), resulting in a higher concentration of macro- and micro-nutrients within the food, including cholesterol. However, as reported in Table 2, the tannins significantly (p < 0.05) affected the content of cholesterol in the S pasta shape, while no significant differences (p > 0.05) were found in the F pasta shape. Moreover, while tannin A impacted the cholesterol content only in uncooked pasta, tannin B significantly (p < 0.01) affected cholesterol occurrence in both uncooked and cooked pasta. Thus, the pasta shape as well as the kind of tannin seems to influence the retention of cholesterol in pasta. This is not unexpected, as it is well known that the success of adding bioactive compounds into food products in terms of not only antioxidant activity but also of food quality and safety depends on both the type of bioactive compound (and specifically its molecular structure) and the food matrix (Sabaghi et al., 2022).
On the other hand, cholesterol complexing activity was in deep investigated in egg yolk. For instance, Lamas et al. (2016) suggested the use of chitosan as promising strategy to reduce the presence of cholesterol in egg-derived foods. However, no data about cholesterol complexing activity with tannins has been reported before. These results reported in the present paper, for the first time, demonstrated how the use of appropriate tannin could contribute to reduce the presence of cholesterol in egg pasta. That phenomenon could be ascribed to tannin and cholesterol coprecipitation through ionic and hydrophobic interactions as well as intermolecular hydrogen bonds (Zeng et al., 2020). However, since the coprecipitation may impact on cholesterol water solubility, the cholesterol content in pasta cooking water was also investigated (“Cholesterol Content in Cooking Water”). As stated above, the cooking process did not significantly (p > 0.05) affect the content of cholesterol in pasta except for tannin B added in S shape; in fact, cooked samples added by 0.50 and 1.00% of tannin B displayed a cholesterol decrease by 18% and 33%, respectively (Supplementary Table S2). Probably, that behavior could be due to a migration phenomenon of tannin-sterol complex from pasta to cooking water as the hydrophilicity of tannin tends to prevail as reported in the “Cholesterol Content in Cooking Water” section.
Cholesterol Content in Cooking Water
In order to confirm the hypothesis of cholesterol transfer from the pasta to the cooking water, the latter was analyzed in terms of total cholesterol content. As reported in Table 3, the cooking water of S pasta shape containing tannin B displayed an increased amount of cholesterol as related to the tannin concentration. The formulation of pasta containing at least 0.50% of tannin B in S shape increased the solubility of cholesterol in the cooking water. The cooking water of control samples displayed a cholesterol content about 0.35 μg/mL of water for both shapes and then remained constant when tannin A was tested (p > 0.05). In contrast, in the case of tannin B in the S shape, a significant rise in cholesterol content was observed as the percentage of fortification increased, reaching a value of 0.56 ± 0.03 and 1.04 ± 0.05 μg/mL when tannin was added at 0.50 and 1.00%, respectively. In order to better explain that phenomenon, two hypotheses have been considered. Firstly, a synergistic effect between the type of tannin and the shape of pasta, which is distinguished by a specific and characteristic microstructure, could be involved in the mechanism of cholesterol loss during cooking. Food microstructure can be defined as the organization, spatial arrangement, and interaction of food constituents resulting in a particular microscopically visible spatial partition of different material phases (Verboven et al., 2018). The microscopic organization of food products has a great impact on their nutritional value since it contributes both to the binding of food macromolecules and to the protection of easily degradable components from structural changes during processing (Parada & Aguilera, 2007). According to the literature, S and F pasta shapes own different microstructures (Aravind et al., 2012; Martín-Esparza et al., 2018) since S shape is reported to be much more outlined and developed than F shape. Thus, the interaction between the components inside the pasta could be different, leading to a link between cholesterol and tannin more or less encouraged. In addition, the greater solubilization of cholesterol within the cooking water could be ascribed to the greater capacity of the water to penetrate into S shape for higher interspace volume, combined with potential surfactant activity, particularly by gallic acid; however, a more in deep investigation is required to better understand how the tannins are able to conjugate or complex cholesterol molecules related to its different forms (free vs esterified). Another explanation may be related to the formation of a surface crust during dry processing due to excessive moisture loss (Migliori et al., 2005). In fact, during the drying phase, a preliminary dehydration of the surface of the dough takes place with the formation of a thin crust with the aim of preventing pasta from sticking or becoming deformed. However, the shape and dimension are parameters that determine a different transfer of moisture from pasta to the air (Conte et al., 2021). Consequently, due to the greater surface exposure of F shape to air, the transfer of moisture to the outside could be faster and the formation of the crust easier than for the S shape, where moisture is better retained inside. Thus, the penetration of water inside the F shape pasta during the cooking step could be reduced resulting in a lower cholesterol solubility.
Cholesterol Oxidation Products (COPs)
Finally, it has been verified the antioxidant activity of tannins in reduction or inhibition of cholesterol oxidation, representing a suitable valid solution to be applied in processed foods containing ingredients with a high content of cholesterol. The COPs determined in the pasta samples were 7α-hydroxycholesterol (0.0722–0.1890 μg/g of pasta; 7α-HC), 7β-hydroxycholesterol (0.0491–0.2183 μg/g of pasta; 7β-HC), and 7-ketocholesterol (0.1030–0.4432 μg/g of pasta; 7-KC). In addition, 5,6 α/β-epoxycholesterol isomers, triol, and 25-hydroxycholesterol were detected in trace amounts. The total amount of COPs was ranged from 0.16 ± 0.01 to 0.66 ± 0.11 μg/g of dry pasta (Table 4).
Figure 4 shows the content of COPs in the pasta samples. Significant differences (p < 0.05) were found in uncooked pasta as related to the type of tannin. Within F shape, as the amount of tannin B increased up to 1.00%, COPs’ content decreased up to 60%. On the other hand, in S pasta shape, only 1.00% of tannin B significantly reduced the amount of COPs. On the contrary, tannin A led to a significant increase of COPs in both shapes. In F shape, a significant COPs increment was found (p < 0.05) rising from 0.41 μg/g (control) to 0.66 μg/g (0.25% tannin concentration), while in the S shape an increase was observed with respect to control as the concentration of tannin increased (p < 0.05). However, in order to better define the extent of cholesterol oxidation with respect to the total cholesterol, the oxidation factor (Fox; %) was calculated. The tannin B added at 1.00% shown the lowest Fox on both pasta shapes F (0.5%) and S (0.7%) confirming the tannin B as greater antioxidant tannin. On the other hand, F pasta shape samples with tannin A displayed higher value of Fox (2.1%) with respect to control (1.8%).
Total cholesterol oxidation products (COPs; μg/g dry pasta) in cooked squared (S) and rectangular shape (F) added with tannins A and B. Each bar represents the mean ± standard deviation of three independent experiments (n = 3). Different letters denote statistically different means (Tukey’s test p < 0.05). Significance: *p < 0.05
From the results obtained, it seems that tannin A shows a pro-oxidant activity. As reported by Salminen et al. (2011), there are several studies reporting controversial results regarding the activities of tannins, referring that the type, dosage, and matrix into which they are added, may be determining factors in the balance between beneficial and deleterious effects. In that case, the pasteurized egg product affects the pH of the dough. In fact, as reported by Atilgan and Unluturk (2008), the pH values of the egg product vary with temperature, due to the nature of the chemical composition and the ionic mobility in the liquid. Thus, the pH decreases because ionic mobility increases with increasing temperature. In formulating the dough, the egg product was stored at refrigeration temperature and used at room temperature, thus estimating a pH between 7.96 and 8.00 (Atilgan & Unluturk, 2008). The high pH value and co-presence of tannin A, based on ellagitannins, probably resulted in a pro-oxidant activity. Direct pro-oxidant activities are based on the generation of a phenoxyl radical or a redox complex with a transition metal ion. Phenoxyl radicals can react with oxygen to generate O2−, H2O2, and a complex combination of semiquinones and quinones, leading to an increase in free radicals present in the ambient, increasing the oxidation reactions (Barbehenn et al., 2006; Moilanen et al., 2016). On the other hand, authors reported a superior pro-oxidant activity of ellagitannin with respect to simple gallic acid derivatives, gallotannins, or proanthocyanidins (Eghbaliferiz & Iranshahi, 2016). Finally, the effect of cooking on the generation of COPs was evaluated (Supplementary Table S3) and no significant differences were found (p > 0.05), indicating that, despite the exposure to high temperatures, 4 min of cooking are not sufficient to trigger the oxidation of cholesterol in significant way.
Principal Component Analysis (PCA) of All Data
All data for phenolic compounds, radical scavenging activity, cholesterol, and COPs content of raw and cooked egg pasta were submitted to PCA to better understand which parameters were the most significant for analyzing the impact of tannins, their concentration, and pasta shape on egg pasta.
The total variability for the first two principal components was estimated to be 73.02%. As reported in Fig. 5, the 7α-HC, 7β-HC, 7-KC, and total COPs were more correlated to PC1, which explained the 49.20% of total variance and fully separated from TPC and RSA. Moreover, the total cholesterol found in egg pasta as well as in cooking water was mostly correlated with PC2, with a variance of 23.82%. The PCA score plot is shown in Fig. 6. Tannins A and B used at high concentrations were fully recognized. In particular, tannin A added in both pasta shapes resulted more characterized by all COPs, while egg pasta formulated with the highest concentration level of tannin B was located in the same cluster with TPC, RSA, and total cholesterol found in both egg pasta and cooking water. These results confirm the antioxidant effect of gallotannin and its impact on cholesterol water solubility.
Conclusions
In the present study, the effect of ellagic acid esters of glucose (ellagitannins) and gallic acid esters of quinic acid (gallotannins) on the content of cholesterol and oxysterols in egg pasta was investigated also considering the contribute of pasta shape. The results demonstrated that tannin based on gallic acid ester was the most performing in egg pasta, with a substantial increase in both TPC and RSA without significant differences due to different pasta shapes considered. Again, the high antiradical activity of gallotannins suggests its use as natural antioxidant able to reduce cholesterol oxidation. In general, the results highlighted that tannins interacted with cholesterol affecting its content in egg pasta. However, without the use of tannins, F pasta shape collected higher cholesterol than S pasta shape. Furthermore, due to coprecipitation or surfactant properties of tannins, the gallotannins in the S pasta shape improved the solubility of cholesterol in water cooking revealing interesting activity to be in deep investigated. To our knowledge, no results were reported before about the hypocholesterolemic effect of gallotannins, suggesting its use as promising technological strategy for the development of more healthy foods.
In terms of cholesterol oxidative behavior, as shown for TPC and RSA, gallotannin was more effective than ellagitannin in reducing the occurrence of COPs in both egg pasta shapes. The current study confirms that gallotannins may be a promising alternative to synthetic antioxidants for developing fresh egg pasta with a low content of COPs; however, further research is needed to better understand how tannins can bind or interact with cholesterol impacting its water solubility also taking into account both free and esterified forms.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Hijazeen, M., Lee, E., Mendonca, A., & Ahn, D. (2016). Effects of tannic acid on lipid and protein oxidation, color, and volatiles of raw and cooked chicken breast meat during storage. Antioxidants, 5(2), 19. https://doi.org/10.3390/antiox5020019
Alide, T., Wangila, P., & Kiprop, A. (2020). Effect of cooking temperature and time on total phenolic content, total flavonoid content and total in vitro antioxidant activity of garlic. BMC Research Notes, 13(1), 564. https://doi.org/10.1186/s13104-020-05404-8
Ansorena, D., Barriuso, B., Cardenia, V., Astiasarán, I., Lercker, G., & Rodriguez-Estrada, M. T. (2013). Thermo-oxidation of cholesterol: Effect of the unsaturation degree of the lipid matrix. Food Chemistry, 141(3), 2757–2764. https://doi.org/10.1016/j.foodchem.2013.04.129
Aravind, N., Sissons, M. J., Fellows, C. M., Blazek, J., & Gilbert, E. P. (2012). Effect of inulin soluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chemistry, 132(2), 993–1002. https://doi.org/10.1016/j.foodchem.2011.11.085
Atilgan, M. R., & Unluturk, S. (2008). Rheological properties of liquid egg products (LEPS). International Journal of Food Properties, 11(2), 296–309. https://doi.org/10.1080/10942910701329658
Barbehenn, R. V., Jones, C. P., Hagerman, A. E., Karonen, M., & Salminen, J. P. (2006). Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high ph: Potential impact on caterpillars. Journal of Chemical Ecology, 32(10), 2253–2267. https://doi.org/10.1007/s10886-006-9143-7
Barbieri, S., Mercatante, D., Balzan, S., Esposto, S., Cardenia, V., Servili, M., Novelli, E., Taticchi, A., & Rodriguez-Estrada, M. T. (2021). Improved oxidative stability and sensory quality of beef hamburgers enriched with a phenolic extract from olive vegetation water. Antioxidants, 10, 1969. https://doi.org/10.3390/antiox10121969
Barbosa-Pereira, L., Guglielmetti, A., & Zeppa, G. (2018). Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silverskin. Food and Bioprocess Technology, 11(4), 818–835. https://doi.org/10.1007/s11947-017-2045-6
Bianchi, F., Giuberti, G., Cervini, M., & Simonato, B. (2022). Fortification of durum wheat fresh pasta with maqui (Aristotelia chilensis) and its effects on technological, nutritional, sensory properties, and predicted glycemic index. Food and Bioprocess Technology, 15(7), 1563–1572. https://doi.org/10.1007/s11947-022-02838-9
Cantele, C., Rojo-Poveda, O., Bertolino, M., Ghirardello, D., Cardenia, V., Barbosa-Pereira, L., & Zeppa, G. (2020). In vitro bioaccessibility and functional properties of phenolic compounds from enriched beverages based on cocoa bean shell. Foods, 9(6), 715. https://doi.org/10.3390/foods9060715
Cappa, C., & Alamprese, C. (2017). Brewer’s spent grain valorization in fiber-enriched fresh egg pasta production: Modelling and optimization study. Lwt, 82, 464–470. https://doi.org/10.1016/j.lwt.2017.04.068
Cardenia, V., Rodriguez-Estrada, M. T., Baldacci, E., Savioli, S., & Lercker, G. (2012). Analysis of cholesterol oxidation products by fast gas chromatography/mass spectrometry. Journal of Separation Science, 35(3), 424–430. https://doi.org/10.1002/jssc.201100660
Cardenia, V., Rodriguez-Estrada, M. T., Lorenzini, A., Bandini, E., Angeloni, C., Hrelia, S., & Malaguti, M. (2017). Effect of broccoli extract enriched diet on liver cholesterol oxidation in rats subjected to exhaustive exercise. Journal of Steroid Biochemistry & Molecular Biology, 169, 137–144. https://doi.org/10.1016/j.jsbmb.2016.04.005
Chudy, S., & Teichert, J. (2021). Oxysterols in stored powders as potential health hazards. Scientific Reports, 11(1), 21192. https://doi.org/10.1038/s41598-021-00636-5
Conte, P., Piga, A., Del Caro, A., Urgeghe, P. P., & Fadda, C. (2021). Italian dried pasta; conventional and innovative ingredients and processing. In F. Boukid (Ed.), Cereal-Based Foodstuffs: The Backbone of Mediterranean Cuisine (pp. 89–110). Springer Nature.
da Silva Soares, B., de Carvalho, C. W. P., & Garcia-Rojas, E. (2021). Microencapsulation of sacha inchi oil by complex coacervates using ovalbumin-tannic acid and pectin as wall materials. Food and Bioprocess Technology, 14(5), 817–830. https://doi.org/10.1007/s11947-021-02594-2
de Oliveira, V. S., Ferreira, F. S., Cople, M. C. R., Labre, T. D. S., Augusta, I. M., Gamallo, O. D., & Saldanha, T. (2018). Use of natural antioxidants in the inhibition of cholesterol oxidation: A review. Comprehensive Reviews in Food Science and Food Safety, 17(6), 1465–1483. https://doi.org/10.1111/1541-4337.12386
Dutta, D., Nayak, A., & Dutta, D. (2022). Reconnoitring the usage of agroindustrial waste in carotenoid production for food fortification: A sustainable approach to tackle vitamin A deficiency. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-022-02888-z
Eghbaliferiz, S., & Iranshahi, M. (2016). Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytotherapy Research, 30, 1379–1391. https://doi.org/10.1002/ptr.5643
Espinosa, R. R., Inchingolo, R., Alencar, S. M., Rodriguez-Estrada, M. T., & Castro, I. A. (2015). Antioxidant activity of phenolic compounds added to a functional emulsion containing omega-3 fatty acids and plant sterol esters. Food Chemistry, 182, 95–104. https://doi.org/10.1016/j.foodchem.2015.02.130
Fares, C., Platani, C., Baiano, A., & Menga, V. (2010). Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chemistry, 119(3), 1023–1029. https://doi.org/10.1016/j.foodchem.2009.08.006
Folch, J., Lees, M., & Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry, 226(1), 497–509. https://doi.org/10.1016/s0021-9258(18)64849-5
Fraga-Corral, M., García-Oliveira, P., Pereira, A. G., Lourenço-Lopes, C., Jimenez-Lopez, C., Prieto, M. A., & Simal-Gandara, J. (2020). Technological application of tannin-based extracts. Molecules, 25(3), 614. https://doi.org/10.3390/molecules25030614
Fraga-Corral, M., Otero, P., Echave, J., Garcia-Oliveira, P., Carpena, M., Jarboui, A., Nuñez-Estevez, B., Simal-Gandara, J., & Prieto, M. A. (2021). By-products of agri-food industry as tannin-rich sources: A review of tannins’ biological activities and their potential for valorization. Foods, 10(1), 137. https://doi.org/10.3390/foods10010137
Fruet, A. P. B., Giotto, F. M., Fonseca, M. A., Nörnberg, J. L., & De Mello, A. S. (2020). Effects of the incorporation of tannin extract from quebracho colorado wood on color parameters, lipid oxidation, and sensory attributes of beef patties. Foods, 9(5), 667. https://doi.org/10.3390/foods9050667
Gadow, A. V., Joubert, E., & Hansmann, C. F. (1997). Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea. Food Chemistry, 60(1), 73–77. https://doi.org/10.1016/S0308-8146(96)00312-3
Garcia-Amezquita, L. E., Tejada-Ortigoza, V., Serna-Saldivar, S. O., & Welti-Chanes, J. (2018). Dietary fiber concentrates from fruit and vegetable by-products: Processing, modification, and application as functional ingredients. Food and Bioprocess Technology, 11(8), 1439–1463. https://doi.org/10.1007/s11947-018-2117-2
Gooding, H. C., & de Ferranti, S. D. (2010). Cardiovascular risk assessment and cholesterol management in adolescents: Getting to the heart of the matter. Current Opinion in Pediatrics, 22(4), 398–404. https://doi.org/10.1097/MOP.0b013e32833a6e22
Gülçin, I., Huyut, Z., Elmastaş, M., & Aboul-Enein, H. Y. (2010). Radical scavenging and antioxidant activity of tannic acid. Arabian Journal of Chemistry, 3(1), 43–53. https://doi.org/10.1016/j.arabjc.2009.12.008
Gunathilake, K. D. P. P., Somathilaka Ranaweera, K. K. D., & Vasantha Rupasinghe, H. P. (2018). Effect of different cooking methods on polyphenols, carotenoids and antioxidant activities of selected edible leaves. Antioxidants, 7(9), 117. https://doi.org/10.3390/antiox7090117
Karagozlu, M., Ocak, B., & Ocak, Ö. (2021). Effect of tannic acid concentration on the physicochemical, thermal, and antioxidant properties of gelatin/gum arabic–walled microcapsules containing Origanum onites L. essential oil. Food and Bioprocess Technology, 14(7), 1231–1243. https://doi.org/10.1007/s11947-021-02633-y
Karim, M. A., Rahman, M. M., Pham, N. D., & Fawzia, S. (2017). Food microstructure as affected by processing and its effect on quality and stability. In S. Devahastin (Ed.), Food Microstructure and Its Relationship with Quality and Stability (pp. 43–57). Elsevier. https://doi.org/10.1016/B978-0-08-100764-8.00003-4
Kazemi, M., Karim, R., Mirhosseini, H., Hamid, A. A., & Tamnak, S. (2017). Processing of parboiled wheat noodles fortified with pulsed ultrasound pomegranate (Punica granatum L. var. Malas) peel extract. Food and Bioprocess Technology, 10(2), 379–393. https://doi.org/10.1007/s11947-016-1825-8
Kloudova, A., Guengerich, F. P., & Soucek, P. (2017). The role of oxysterols in human cancer. Trends in Endocrinology & Metabolism, 28(7), 485–496. https://doi.org/10.1016/j.tem.2017.03.002
Kuczyńska, A., Cardenia, V., Ogrodowicz, P., Kempa, M., Rodriguez-Estrada, M. T., & Mikołajczak, K. (2019). Effects of multiple abiotic stresses on lipids and sterols profile in barley leaves (Hordeum vulgare L.). Plant Physiology and Biochemistry, 141, 215–224. https://doi.org/10.1016/j.plaphy.2019.05.033
Kumar, N., & Goel, N. (2019). Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnology Reports, 24, e00370. https://doi.org/10.1016/j.btre.2019.e00370
Lamas, A., Anton, X., Miranda, J. M., Roca-Saavedra, P., Cardelle-Cobas, A., Ibarra, I. S., Franco, C. M., & Cepeda, A. (2016). Technological strategies for the development of egg-derived products with reduced content of cholesterol. Food and Bioprocess Technology, 9(1), 81–90. https://doi.org/10.1007/s11947-015-1599-4
Lamy, E., Pinheiro, C., Rodrigues, L., Capela e Silva, F., Lopes, O. S., Tavares, S., & Gaspar, R. (2016). Determinants of tannin-rich food and beverage consumption: Oral perception vs. psychosocial aspects. In C. A. Combs (Ed.), Tannins: Biochemistry, Food Sources and Nutritional Properties (pp. 29–58). Nova Science Publishers.
Lisiecka, K., Wójtowicz, A., Dziki, D., & Gawlik-Dziki, U. (2019). The influence of Cistus incanus L. leaves on wheat pasta quality. Journal of Food Science and Technology, 56(9), 4311–4322. https://doi.org/10.1007/s13197-019-03900-9
Luise, D., Cardenia, V., Zappaterra, M., Motta, V., Bosi, P., Rodriguez-Estrada, M. T., & Trevisi, P. (2018). Evaluation of breed and parity order effects on the lipid composition of porcine colostrum. Journal of Agricultural and Food Chemistry, 66(49), 12911–12920. https://doi.org/10.1021/acs.jafc.8b03097
Malaguti, M., Cardenia, V., Rodriguez-Estrada, M. T., & Hrelia, S. (2019). Nutraceuticals and physical activity: Their role on oxysterols-mediated neurodegeneration. Journal of Steroid Biochemistry and Molecular Biology, 193, 105430. https://doi.org/10.1016/j.jsbmb.2019.105430
Martín-Esparza, M., Raga, A., González-Martínez, C., & Albors, A. (2018). Micronised bran-enriched fresh egg tagliatelle: Significance of gums addition on pasta technological features. Food Science and Technology International, 24(4), 309–320. https://doi.org/10.1177/1082013217750683
Migliori, M., Gabriele, D., de Cindio, B., & Pollini, C. M. (2005). Modelling of high quality pasta drying: Quality indices and industrial application. Journal of Food Engineering, 71(3), 242–251. https://doi.org/10.1016/j.jfoodeng.2004.11.004
Minatel, I. O., Borges, C. V., Ferreira, M. I., Gomez, H. A. G., Chen, C.-Y.O., & Lima, G. P. P. (2017). Phenolic compounds: Functional properties, impact of processing and bioavailability. In M. S. Hernandez (Ed.), Phenolic Compounds - Biological Activity. InTech. https://doi.org/10.5772/66368
Moilanen, J., Karonen, M., Tähtinen, P., Jacquet, R., Quideau, S., & Salminen, J. P. (2016). Biological activity of ellagitannins: Effects as anti-oxidants, pro-oxidants and metal chelators. Phytochemistry, 125, 65–72. https://doi.org/10.1016/j.phytochem.2016.02.008
Moreno, C. R., Fernández, P. C. R., Rodríguez, E. O. C., Carrillo, J. M., & Rochín, S. M. (2018). Changes in nutritional properties and bioactive compounds. In S. Z. Qamar (Ed.), Cereals during extrusion cooking in extrusion of metals, polymers and food products (pp. 103–124). InTechOpen. https://doi.org/10.5772/intechopen.68753
Mortensen, M. B., & Nordestgaard, B. G. (2020). Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: A contemporary primary prevention cohort. The Lancet, 396(10263), 1644–1652. https://doi.org/10.1016/S0140-6736(20)32233-9
Nelson, R. H. (2013). Hyperlipidemia as a risk factor for cardiovascular disease. Primary Care - Clinics in Office Practice, 40(1), 195–211. https://doi.org/10.1016/j.pop.2012.11.003
Parada, J., & Aguilera, J. M. (2007). Food microstructure affects the bioavailability of several nutrients. Journal of Food Science, 72(2), 21–32. https://doi.org/10.1111/j.1750-3841.2007.00274.x
Poli, G., Leoni, V., Biasi, F., Canzoneri, F., Risso, D., & Menta, R. (2022). Oxysterols: from redox bench to industry. Redox Biology, 49, 102220. https://doi.org/10.1016/j.redox.2021.102220
Presidential Decree. (2001). n. 187, Regolamento per la revisione della normativa sulla produzione e commercializzazione di sfarinati e paste alimentari, a norma dell’articolo 50 della legge 22 febbraio 1994, n. 146. Gazzetta Ufficiale della Repubblica Italiana. n. 117 of 05-22-2001.
Pugnaloni, S., Alia, S., Gabrielli, M., Di Paolo, A., Turco, I., Mazzanti, L., Orsini, R., Vignini, A., & Ferretti, G. (2021). Senatore cappelli (Triticum turgidum spp. durum) pasta: A study on the nutritional quality of whole grains and its physical form. International Journal of Food Sciences and Nutrition, 73(4), 451–459. https://doi.org/10.1080/09637486.2021.2025212
Rose-Sallin, C., Huggett, A. C., Bosset, J. O., Tabacchi, R., & Fay, L. B. (1995). Quantification of cholesterol oxidation products in milk powders using [2H7] cholesterol to monitor cholesterol autoxidation artifacts. Journal of Agricultural and Food Chemistry, 43(4), 935–941. https://doi.org/10.1021/jf00052a017
Sabaghi, M., Tavasoli, S., Jamali, S. N., Katouzian, I., & Faridi Esfanjani, A. (2022). The pros and cons of incorporating bioactive compounds within food networks and food contact materials: A review. Food and Bioprocess Technology, 15, 2422–2455. https://doi.org/10.1007/s11947-022-02837-w
Salminen, J. P., Karonen, M., & Sinkkonen, J. (2011). Chemical ecology of tannins: Recent developments in tannin chemistry reveal new structures and structure-activity patterns. Chemistry - A European Journal, 17(10), 2806–2816. https://doi.org/10.1002/chem.201002662
Sęczyk, Ł, Gawlik-Dziki, U., & Świeca, M. (2021). Influence of phenolic-food matrix interactions on in vitro bioaccessibility of selected phenolic compounds and nutrients digestibility in fortified white bean paste. Antioxidants, 10(11), 1825. https://doi.org/10.3390/antiox10111825
Sewoski, S., Veiko, A., Olchowik-Grabarek, E., Dubis, A., Wilczewska, A. Z., Markiewicz, K. H., Zavodnik, I. B., Lapshina, E., Dobrzynska, I., Abdulladjanova, N., & Zamaraeva, M. (2022). Hydrolysable tannins change physicochemical parameters of lipid nano-vesicles and reduce DPPH radical – experimental studies and quantum chemical analysis. BBA – Biomembranes, 1864, 183778.
Sharma, K., Babaei, A., Oberoi, K., Aayush, K., Sharma, R., & Sharma, S. (2022). Essential oil nanoemulison edible coating in food industry: A review. Food and Bioprocess Technology, 15, 2375–2395. https://doi.org/10.1007/s11947-022-02811-6
Simonato, B., Trevisan, S., Tolve, R., Favati, F., & Pasini, G. (2019). Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. Lwt, 114, 108368. https://doi.org/10.1016/j.lwt.2019.108368
Singleton, V. L., & Rossi, J. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Smeriglio, A., Barreca, D., Bellocco, E., & Trombetta, D. (2017). Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. British Journal of Pharmacology, 174(11), 1244–1262. https://doi.org/10.1111/bph.13630
Srikiatden, J., & Roberts, J. S. (2007). Moisture transfer in solid food materials: A review of mechanisms, models, and measurements. International Journal of Food Properties, 10(4), 739–777. https://doi.org/10.1080/10942910601161672
Suo, X., Mosca, A. C., Pellegrini, N., & Vittadini, E. (2021). Effect of pasta shape and gluten on pasta cooking quality and structural breakdown during mastication. Food and Function, 12(22), 11577–11585. https://doi.org/10.1039/d1fo02339j
Tanaka, T., Matsuo, Y., & Saito, Y. (2018). Solubility of tannins and preparation of oil-soluble derivatives. Journal of Oleo Science, 67(10), 1179–1187. https://doi.org/10.5650/jos.ess18164
Tolve, R., Pasini, G., Vignale, F., Favati, F., & Simonato, B. (2020). Effect of grape pomace addition on the technological, sensory, and nutritional properties of durum wheat pasta. Foods, 9(3), 354. https://doi.org/10.3390/foods9030354
Verardo, V., Riciputi, Y., Messia, M. C., Marconi, E., & Caboni, M. F. (2017). Influence of drying temperatures on the quality of pasta formulated with different egg products. European Food Research and Technology, 243(5), 817–825. https://doi.org/10.1007/s00217-016-2795-9
Verardo, V., Messia, M., Marconi, E., & Caboni, M. F. (2020). Effect of different egg products on lipid oxidation of biscuits. Foods, 9(11), 1714. https://doi.org/10.3390/foods9111714
Verboven, P., Defraeye, T., & Nicolai, B. (2018). Measurement and visualization of food microstructure. In S. Devahastin (Ed.), Food microstructure and its relationship with quality and stability (pp. 3–28). Woodhead Publishing. https://doi.org/10.1016/B978-0-08-100764-8.00001-0
Zeng, X., Du, Z., Ding, X., & Jiang, W. (2020). Characterization of the direct interaction between apple condensed tannins and cholesterol in vitro. Food Chemistry, 309, 125762. https://doi.org/10.1016/j.foodchem.2019.125762
Acknowledgements
The authors thank SilvaTeam S.p.a. for providing tannins.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. The experimental work was supported by the Fund for Local Research (FFO 2019), University of Turin.
Author information
Authors and Affiliations
Contributions
Ambra Bonciolini: investigation, formal analysis, writing–original draft; Carolina Cantele: formal analysis, writing–review; Nicolò Ivan Salgarella: investigation, formal analysis; Giuseppe Zeppa: resources, writing–review and editing; Marta Bertolino: conceptualization, writing–review; Vladimiro Cardenia: conceptualization, writing–review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonciolini, A., Cantele, C., Salgarella, N.I. et al. Effect of Tannins on Cholesterol Content and Its Oxidation in Egg Pasta as Related to Different Pasta Shapes. Food Bioprocess Technol 16, 1541–1554 (2023). https://doi.org/10.1007/s11947-023-03016-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03016-1