Abstract
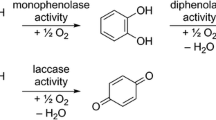
Plants synthesize a diversity of tannin structures but little is known about whether these different types have different oxidative activities in herbivores. Oxidative activities of hydrolyzable and condensed tannins were compared at pH 10 with two methods: EPR spectrometry was used to quantify semiquinone radicals in anoxic conditions and a spectrophotometric assay was used to measure the rate of browning of phenolics oxidized in ambient oxygen conditions. A little-studied group of hydrolyzable tannins (ellagitannins) contained the most active tannins examined, forming high concentrations of semiquinone radicals and browning at the highest rates. On average, galloyl glucoses and high-molecular-weight gallotannins had intermediate to low oxidative activities. Condensed tannins generally formed low levels of semiquinone radicals and browned most slowly. The results suggest that ellagitannin-rich plants have active oxidative defenses against herbivores, such as caterpillars, whereas the opposite may hold true for plants that contain predominantly condensed tannins or high-molecular-weight gallotannins.






Similar content being viewed by others
References
Aerts, R. J., Barry, T. N., and McNabb, W. C. 1999. Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agric. Ecosyst. Environ. 75:1–12.
Appel, H. M. 1993. Phenolics in ecological interactions: the importance of oxidation. J. Chem. Ecol. 19:1521–1552.
Appel, H. M. and Martin, M. M. 1990. Gut redox conditions in herbivorous lepidopteran larvae. J. Chem. Ecol. 16:3277–3290.
Barbehenn, R. V., Cheek, S., Gasperut, A., Lister, E., and Maben, R. 2005. Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midguts of Malacosoma disstria and Orgyia leucostigma caterpillars. J. Chem. Ecol. 31:969–988.
Barbehenn, R. V., Poopat, U., and Spencer, B. 2003. Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem. Mol. Biol. 33:125–130.
Bors, W. and Michel, C. 1999. Antioxidant capacity of flavanols and gallate esters: Pulse radiolysis studies. Free Radic. Biol. Med. 27:1413–1426.
Bors, W., Michel, C., and Stettmaier, K. 2000. Electron paramagnetic resonance studies of radical species of proanthocyanins and gallate esters. Arch. Biochem. Biophys. 374:347–355.
Bors, W., Michel, C., and Stettmaier, K. 2001a. Structure–activity relationships governing antioxidant capacities of plant polyphenols. Methods Enzymol. 335:166–180.
Bors, W., Yeap Foo, L., Hertkorn, N., Michel, C., and Stettmaier, K. 2001b. Chemical studies of proanthocyanidins and hydrolyzable tannins. Antiox. Redox Signal. 3:995–1008.
Cao, G., Sofic, E., and Prior, R. L. 1997. Antioxidant and prooxidant behavior of flavonoids: structure–activity relationships. Free Radic. Biol. Med. 22:749–760.
Chan, T., Galati, G., and O’Brien, P. J. 1999. Oxygen activation during peroxidase catalysed metabolism of flavones or flavanones. Chem.-Biol. Interact. 122:15–25.
Cilliers, J. J. L. and Singleton, V. L. 1989. Nonenzymatic autoxidative phenolic browning reactions in a caffeic acid model system. J. Agric. Food Chem. 37:390–396.
Fukuhara, K., Nakanishi, I., Shimada, T., Ohkubo, K., Miyazaki, K., Hakamata, W., Urano, S., Ozawa, T., Okuda, H., Miyata, N., Ikota, N., and Fukuzumi, S. 2003. A planar catechin analogue as a promising antioxidant with reduced prooxidant activity. Chem. Res. Toxicol. 16:81–86.
Fukumoto, L. R. and Mazza, G. 2000. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 48:3597–3604.
Gant, T. W., Ramakrishna, R., Mason, R. P., and Cohen, G. M. 1988. Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem. Biol. Interact. 65:157–173.
Grace, S. C., Yamasaki, H., and Pryor, W. A. 1999. Spin stabilizing approach to radical characterization of phenylpropanoid antioxidants: an ESR study of chlorogenic acid oxidation in the horseradish peroxidase, tyrosinase, and ferrylmyoglobin protein radical systems, pp. 435–450, in G. G. Gross, R. W. Hemingway, T. Yoshida, and S. J. Branham (eds.). Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology. Kluwer Academic/Plenum Publishers, New York.
Gross, G. G. 1999. Biosynthesis of hydrolyzable tannins, pp. 799–826, in B. M. Pinto (ed.). Comprehensive Natural Products Chemistry, vol. 3, Elsevier, Amsterdam, the Netherlands.
Guo, Q., Zhao, B., Shen, S., Hou, J., Hu, J., and Xin, W. 1999. ESR study on the structure–activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta 1427:13–23.
Hagerman, A. E. and Butler, L. G. 1991. Tannins and lignins, pp. 355–388, in G. A. Rosenthal and M. R. Berenbaum (eds.). Herbivores: Their Interactions with Secondary Plant Metabolites. Second edition. Vol. I: The Chemical Participants. Academic Press, San Diego, CA, USA.
Hagerman, A. E., Riedl, K. M., Jones, G. A., Sovik, K. N., Ritchard, N. T., Hartzfeld, P. W., and Riechel, T. L. 1998. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 40:801–805.
Hagerman, A. E. 1989. Chemistry of tannin–protein complexation, pp. 323–333, in R.W. Hemingway, and J.J. Karchesy, (eds.). Chemistry and Significance of Condensed Tannins. Plenum Publishing Corp., New York, NY, USA.
Hagerman, A. E. and Butler, L. G. 1978. Protein precipitation method for the determination of tannins. J. Agric. Food Chem. 26:809–812.
Hagerman, A. E. and Robbins, C. T. 1993. Specificity of tannin binding salivary proteins relative to diet selection by mammals. Can. J. Zool. 71:628–633.
Hatano, T., Edamatsu, R., Hiramatsu, M., Mori, A., Fujiat, Y., Yasuhara, T., Yoshida, T., and Okuda, T. 1989. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 37:2016–2021.
Hodnick, W. F., Milosavljevic, E. B., Nelson, J. H., and Pardini, R. S. 1988. Relationship between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem. Pharmacol. 37:2607–2611.
Ito, H., Yamaguchi, K., Kim, T.-H., Khennouf, S., Gharzouli, K., and Yoshida, T. 2002. Dimeric and trimeric hydrolyzable tannins from Quercus coccifera and Quercus suber. J. Nat. Prod. 65:339–345.
Johnson, K. S. and Barbehenn, R. V. 2000. Oxygen levels in the gut lumens of herbivorous insects. J. Insect Physiol. 46:897–903.
Jovanovic, S. V., Hara, Y., Steenken, S., and Simic, M. G. 1995. Antioxidant potential of gallocatechins. A pulse radiolysis and laser photolysis study. J. Am. Chem. Soc. 117:9881–9888.
Karonen, M., Loponen, J., Ossipov, V., and Pihlaja, K. 2004. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta 522:105–112.
Karonen, M., Ossipov, V., Sinkkonen, J., Loponen, J., Haukioja, E., and Pihlaja, K. 2006. Quantitative analysis of polymeric proanthocyanidins in birch leaves with normal-phase HPLC. Phytochem. Anal. 17:149–156.
Martin, J. S., Martin, M. M., and Bernays, E. A. 1987. Failure of tannic acid to inhibit digestion or reduce digestibility of plant protein in gut fluids of insect herbivores: implications for theories of plant defense. J. Chem. Ecol. 13:605–621.
McAllister, T. A., Martinez, T., Bae, H. D., Muir, A. D., Yanke, L. J., and Jones, G. A. 2005. Characterization of condensed tannins purified from legume forages: Chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J. Chem. Ecol. 31:2049–2067.
Misra, H. P. and Fridovich, I. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247:3170–3175.
Mukai, K., Mitani, S., Ohara, K., and Nagaoka, S.-I. 2005. Structure–activity relationship of the tocopherol-regeneration reaction by catechins. Free Radic. Biol. Med. 38:1243–1256.
Musso, H. 1967. Phenol coupling, pp. 1–94, in W. I. Taylor and A. R. Battersby (eds.). Oxidative Coupling of Phenols. Marcel Dekker, New York, NY, USA.
Northrup, R. R., Dahlgren, R. A., and McColl, J. G. 1998. Polyphenols as regulators of plant–litter–soil interactions in northern California’s pygmy forest: A positive feedback? Biogeochemistry 42:189–220.
Okuda, T. 1999a. Novel aspects of tannins—renewed concepts and structure–activity relationships. Curr. Org. Chem. 3:609–622.
Okuda, T. 1999b. Antioxidants in herbs: Polyphenols, pp. 393–410, in L. Packer and M. Hiramatsu (eds.). Antioxidant Food Supplements in Human Health. Academic Press, San Diego, CA, USA.
Okuda, T., Yoshida, T., and Hatano, T. 2000. Correlation of oxidative transformations of hydrolyzable tannins and plant evolution. Phytochemistry 55:513–529.
Ossipov, V., Haukioja, E., Ossipova, S., Hanhimäki, S., and Pihlaja, K. 2001. Phenolic and phenolic-related factors as determinants of suitability of mountain birch leaves to an herbivorous insect. Biochem. Syst. Ecol. 29:223–240.
Ossipova, S., Ossipov, V., Haukioja, E., Loponen, J., and Pihlaja, K. 2001. Proanthocyanidins of mountain birch leaves: quantification and properties. Phytochem. Anal. 12:128–133.
Pryor, W. 1986. Oxy-radicals and related species: Their formation, lifetimes, and reactions. Annu. Rev. Physiol. 8:657–667.
Quideau, S., Varadinova, T., Karagiozova, D., Jourdes, M., Pardon, P., Baudry, C., Genova, P., Diakov, T., and Petrova, R. 2004. Main structural and stereochemical aspects of the antiherpetic activity of nonahydroxyterphenoyl-containing C-glycosidic ellagitannins. Chem. Biodiv. 1:247–258.
Rice-Evans, C. A., Miller, N. J., and Paganga, G. 1996 Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20:933–956.
Riipi, M., Ossipov, V., Lempa, K., Haukioja, E., Koricheva, J., Ossipova, S., and Pihlaja, K. 2002. Seasonal changes in birch leaf chemistry: Are there trade-offs between leaf growth and accumulation of phenolics? Oecologia 130:380–390.
Salminen, J.-P. and Lempa, K. 2002. Effects of hydrolysable tannins on a herbivorous insect: fate of individual tannins in insect digestive tract. Chemoecology 12:203–211.
Salminen, J.-P., Ossipov, V., Haukioja, E., and Pihlaja, K. 2001. Seasonal variation in the content of hydrolyzable tannins in leaves of Betula pubescens. Phytochemistry 57:15–22.
Salminen, J.-P., Ossipov, V., Loponen, J., Haukioja, E., and Pihlaja, K. 1999. Characterisation of hydrolyzable tannins from leaves of Betula pubescens by high-performance liquid chromatography–mass spectrometry. J. Chromatogr. A 864:283–291.
Salminen, J.-P., Roslin, T., Karonen, M., Sinkkonen, J., Pihlaja, K., and Pulkkinen, P. 2004. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J. Chem. Ecol. 30:1675–1693.
Simic, M. G. and Jovanovic, S. V. 1994. Inactivation of oxygen radicals by dietary phenolic compounds in anticarcinogenesis, pp. 20–33, in C.-T. Ho, T. Osawa, M.-T. Huang, and R. T. Rosen (eds.). Food Phytochemicals for Cancer Prevention II. American Chemical Society, Washington DC, USA.
Summers, C. B. and Felton, G. W. 1994. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 24:943–953.
Swain, T. 1979. Tannins and lignins, pp. 657–682, in G. A. Rosenthal and D. H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York, NY, USA.
Thiboldeaux, R. L., Lindroth, R. L. and Tracy, J. W. 1998. Effects of juglone (5-hydroxy-1,4-naphthoquinone) on midgut morphology and glutathione status in Saturniid moth larvae. Comp. Biochem. Physiol. 120:481–487.
Vuorela, S., Kreander, K., Karonen, M., Nieminen, R., Hämäläinen, M., Galkin, A., Laitinen, L., Salminen, J.-P., Moilanen, E., Pihlaja, K., Vuorela, H., Vuorela, P., and Heinonen, M. 2005. Preclinical evaluation of rapeseed, raspberry, and pine bark phenolics for health related effects. J. Agric. Food Chem. 53:5922–5931.
Waite, J. H. 1976. Calculating extinction coefficients for enzymatically produced O-quinones. Anal. Biochem. 75:211–218.
Wilkinson, L. 2000. SYSTAT: The system for Statistics. SYSTAT, Inc, Evanston, IL.
Wright, J. S., Carpenter, D. J., McKay, D. J., and Ingold, K. U. 1997. Theoretical calculation of substituent effects on the O–H bond strength of phenolic antioxidants related to vitamin E. J. Am. Chem. Soc. 119:4245–4252.
Yoshioka, H., Sugiura, K., Kawahara, R., Fujita, T., Makino, M., Kamiya, M., and Tsuyuma, S. 1991. Formation of radicals and chemiluminescence during the autoxidation of tea catechins. Agric. Biol. Chem. 55:2717–2723.
Acknowledgments
This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service grant number 2004-35302-14940 to R. Barbehenn and C.P. Constabel. Support for J.-P. Salminen was provided by grant number 204209 from the Academy of Finland. Jonna Kenttä, Riitta Koivikko, Maria Lahtinen, Jaana Liimatainen, Tuuli Luomahaara, Angelica Preetz, and Victor Turhanen assisted with the isolation of tannins. We thank Michael M. Martin for suggesting revisions to this paper, and Jari Sinkkonen for drawing the chemical structures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbehenn, R.V., Jones, C.P., Hagerman, A.E. et al. Ellagitannins have Greater Oxidative Activities than Condensed Tannins and Galloyl Glucoses at High pH: Potential Impact on Caterpillars. J Chem Ecol 32, 2253–2267 (2006). https://doi.org/10.1007/s10886-006-9143-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9143-7