Abstract
Summary
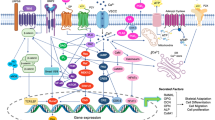
Voltage-sensitive calcium channels (VSCCs) are ubiquitous multimeric protein complexes that are necessary for the regulation of numerous physiological processes. VSCCs regulate calcium influx and various intracellular processes including muscle contraction, neurotransmission, hormone secretion, and gene transcription, with function specificity defined by the channel’s subunits and tissue location.
The functions of VSCCs in bone are often overlooked since bone is not considered an electrically excitable tissue. However, skeletal homeostasis and adaptation relies heavily on VSCCs. Inhibition or deletion of VSCCs decreases osteogenesis, impairs skeletal structure, and impedes anabolic responses to mechanical loading.
Recent Findings
While the functions of VSCCs in osteoclasts are less clear, VSCCs have distinct but complementary functions in osteoblasts and osteocytes.
Purpose of Review
This review details the structure, function, and nomenclature of VSCCs, followed by a comprehensive description of the known functions of VSCCs in bone cells and their regulation of bone development, bone formation, and mechanotransduction.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57(4):411–25. https://doi.org/10.1124/pr.57.4.5.
Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004(253):re15. doi:https://doi.org/10.1126/stke.2532004re15.
Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 2003;13(3):298–307. doi:S0959438803000667 [pii].
Garcia K, Nabhani T, Garcia J. The calcium channel alpha2/delta1 subunit is involved in extracellular signalling. J Physiol. 2008;586(3):727–38. https://doi.org/10.1113/jphysiol.2007.147959.
Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987;84(15):5478–82. https://doi.org/10.1073/pnas.84.15.5478.
Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel beta subunit. J Biol Chem. 1993;268(17):12359–66.
Hullin R, Singer-Lahat D, Freichel M, Biel M, Dascal N, Hofmann F, et al. Calcium channel beta subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 1992;11(3):885–90.
Perezreyes E, Schneider T. Calcium channels - structure, function, and classification. Drug Develop Res. 1994;33(3):295–318. doi:DOI https://doi.org/10.1002/ddr.430330311.
Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368(6466):67–70. https://doi.org/10.1038/368067a0.
Ellinor PT, Zhang JF, Randall AD, Zhou M, Schwarz TL, Tsien RW, et al. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363(6428):455–8. https://doi.org/10.1038/363455a0.
Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, et al. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992;8(1):71–84. https://doi.org/10.1016/0896-6273(92)90109-q.
Varadi G, Lory P, Schultz D, Varadi M, Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991;352(6331):159–62. https://doi.org/10.1038/352159a0.
De Waard M, Pragnell M, Campbell KP. Ca2+ channel regulation by a conserved beta subunit domain. Neuron 1994;13(2):495–503. doi:0896-6273(94)90363-8 [pii].
Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, et al. Importance of the different beta subunits in the membrane expression of the alpha1A and alpha2 calcium channel subunits: studies using a depolarization-sensitive alpha1A antibody. Eur J Neurosci. 1997;9(4):749–59. https://doi.org/10.1111/j.1460-9568.1997.tb01423.x.
Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y., de Waard M. The I-II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron 2000;25(1):177–190. doi:S0896-6273(00)80881-8 [pii].
Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35(6):599–620. https://doi.org/10.1023/b:jobb.0000008026.37790.5a.
Chu PJ, Robertson HM, Best PM. Calcium channel gamma subunits provide insights into the evolution of this gene family. Gene 2001;280(1–2):37–48. doi:S0378-1119(01)00738-7 [pii].
Burgess DL, Gefrides LA, Foreman PJ, Noebels JL. A cluster of three novel Ca2+ channel gamma subunit genes on chromosome 19q13.4: evolution and expression profile of the gamma subunit gene family. Genomics 2001;71(3):339–350. doi:https://doi.org/10.1006/geno.2000.6440 S0888-7543(00)96440-1 [pii].
Klugbauer N, Dai S, Specht V, Lacinova L, Marais E, Bohn G et al. A family of gamma-like calcium channel subunits. FEBS Lett 2000;470(2):189–197. doi:S0014-5793(00)01306-5 [pii].
Rousset M, Cens T, Restituito S, Barrere C, Black JL 3rd, McEnery MW, et al. Functional roles of gamma2, gamma3 and gamma4, three new Ca2+ channel subunits, in P/Q-type Ca2+ channel expressed in Xenopus oocytes. J Physiol. 2001;532(Pt 3):583–93. https://doi.org/10.1111/j.1469-7793.2001.0583e.x.
Moss FJ, Viard P, Davies A, Bertaso F, Page KM, Graham A, et al. The novel product of a five-exon stargazin-related gene abolishes Ca(V)2.2 calcium channel expression. EMBO J. 2002;21(7):1514–23. https://doi.org/10.1093/emboj/21.7.1514.
Arikkath J, Chen CC, Ahern C, Allamand V, Flanagan JD, Coronado R, Gregg RG, Campbell KP Gamma 1 subunit interactions within the skeletal muscle L-type voltage-gated calcium channels. J Biol Chem 2003;278(2):1212–1219. doi:https://doi.org/10.1074/jbc.M208689200 M208689200 [pii].
Kang MG, Chen CC, Felix R, Letts VA, Frankel WN, Mori Y, Campbell KP Biochemical and biophysical evidence for gamma 2 subunit association with neuronal voltage-activated Ca2+ channels. J Biol Chem 2001;276(35):32917–32924. doi:https://doi.org/10.1074/jbc.M100787200 M100787200 [pii].
Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J Neurosci. 1999;19(2):684–91.
Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21(16):6095–104.
Qin N, Yagel S, Momplaisir ML, Codd EE, D'Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Molecular Pharmacology. 2002;62(3):485–96. doi:UNSP 1542/1003845 DOI https://doi.org/10.1124/mol.62.3.485.
Wycisk KA, Zeitz C, Feil S, Wittmer M, Forster U, Neidhardt J et al. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am J Hum Genet. 2006;79(5):973–7. doi:Doi https://doi.org/10.1086/508944.
Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. J Biol Chem. 1991;266(5):3287–93.
Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, et al. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci U S A. 2010;107(4):1654–9. https://doi.org/10.1073/pnas.0908735107.
Wu J, Yan Z, Li Z, Yan C, Lu S, Dong M et al. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 2015;350(6267):aad2395. doi:https://doi.org/10.1126/science.aad2395.
Bannister RA, Beam KG. Ca(V)1.1: the atypical prototypical voltage-gated Ca(2)(+) channel. Biochim Biophys Acta. 2013;1828(7):1587–97. https://doi.org/10.1016/j.bbamem.2012.09.007.
Dolphin AC. The alpha2delta subunits of voltage-gated calcium channels. Biochim Biophys Acta. 2013;1828(7):1541–9. https://doi.org/10.1016/j.bbamem.2012.11.019.
Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13(10):3369–87. https://doi.org/10.1091/mbc.e02-05-0259.
Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102(32):11230–5. https://doi.org/10.1073/pnas.0504183102.
Tran-Van-Minh A, Dolphin AC. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J Neurosci. 2010;30(38):12856–67. https://doi.org/10.1523/JNEUROSCI.2700-10.2010.
Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271(10):5768–76. https://doi.org/10.1074/jbc.271.10.5768.
Davies A, Douglas L, Hendrich J, Wratten J. Tran Van Minh A, Foucault I et al. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J Neurosci. 2006;26(34):8748–57. https://doi.org/10.1523/JNEUROSCI.2764-06.2006.
Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103(46):17537–42. https://doi.org/10.1073/pnas.0409066103.
Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, et al. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42(3):353–66. https://doi.org/10.1016/s0028-3908(01)00181-2.
Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105(9):3628–33. https://doi.org/10.1073/pnas.0708930105.
Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–56. https://doi.org/10.1146/annurev.ne.13.030190.002005.
Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3(8):a003947. https://doi.org/10.1101/cshperspect.a003947.
Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol. 2016;594(19):5369–90. https://doi.org/10.1113/JP272262.
Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA Nomenclature of voltage-gated calcium channels. Neuron 2000;25(3):533–535. doi:S0896-6273(00)81057-0 [pii].
Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316(6027):440–3. https://doi.org/10.1038/316440a0.
Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301(5901):569–74. https://doi.org/10.1038/301569a0.
Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310(5977):501–2. https://doi.org/10.1038/310501a0.
Swandulla D, Armstrong CM. Fast-deactivating calcium channels in chick sensory neurons. J Gen Physiol. 1988;92(2):197–218. https://doi.org/10.1085/jgp.92.2.197.
Fedulova SA, Kostyuk PG, Veselovsky NS. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985;359:431–46. https://doi.org/10.1113/jphysiol.1985.sp015594.
Lacinova L, Klugbauer N, Hofmann F. Low voltage activated calcium channels: from genes to function. Gen Physiol Biophys. 2000;19(2):121–36.
Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–61. https://doi.org/10.1152/physrev.00018.2002.
Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M NNC 55-0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluo ro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther 2004;309(1):193–199. doi:https://doi.org/10.1124/jpet.103.060814 jpet.103.060814 [pii].
McCleskey EW, Fox AP, Feldman DH, Cruz LJ, Olivera BM, Tsien RW, et al. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987;84(12):4327–31. https://doi.org/10.1073/pnas.84.12.4327.
Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends in Neurosciences. 1988;11(10):431–8. doi:Doi https://doi.org/10.1016/0166-2236(88)90194-4.
Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15(4):2995–3012.
Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9(1):85–95. https://doi.org/10.1016/0896-6273(92)90223-z.
Bergh JJ, Shao Y, Puente E, Duncan RL, Farach-Carson MC. Osteoblast Ca(2+) permeability and voltage-sensitive Ca(2+) channel expression is temporally regulated by 1,25-dihydroxyvitamin D(3). Am J Physiol Cell Physiol. 2006;290(3):C822–31.
Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45(4):682–92. https://doi.org/10.1016/j.bone.2009.06.010.
Thompson WR, Majid AS, Czymmek KJ, Ruff AL, Garcia J, Duncan RL, et al. Association of the alpha(2)delta(1) subunit with Ca(v)3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. J Bone Miner Res. 2011;26(9):2125–39. https://doi.org/10.1002/jbmr.437.
Chesnoy-Marchais D, Fritsch J. Voltage-gated sodium and calcium currents in rat osteoblasts. J Physiol. 1988;398:291–311.
Guggino SE, Lajeunesse D, Wagner JA, Snyder SH. Bone remodeling signaled by a dihydropyridine- and phenylalkylamine-sensitive calcium channel. Proc Natl Acad Sci U S A. 1989;86(8):2957–60. https://doi.org/10.1073/pnas.86.8.2957.
Grygorczyk C, Grygorczyk R, Ferrier J. Osteoblastic cells have L-type calcium channels. Bone and mineral. 1989;7(2):137–48.
Gu Y, Preston MR, el Haj AJ, Hamid J, Zamponi GW, Howl J, et al. Osteoblasts derived from load-bearing bones of the rat express both L- and T-like voltage-operated calcium channels and mRNA for alpha 1C, alpha 1D and alpha 1G subunits. Pflugers Arch. 1999;438(4):553–60.
el Haj AJ, Walker LM, Preston MR, Publicover SJ. Mechanotransduction pathways in bone: calcium fluxes and the role of voltage-operated calcium channels. Medical & biological engineering & computing. 1999;37(3):403–9.
Loza JC, Carpio LC, Bradford PG, Dziak R. Molecular characterization of the alpha1 subunit of the L type voltage calcium channel expressed in rat calvarial osteoblasts. J Bone Miner Res. 1999;14(3):386–95.
Barry EL. Expression of mRNAs for the alpha 1 subunit of voltage-gated calcium channels in human osteoblast-like cell lines and in normal human osteoblasts. Calcif Tissue Int. 2000;66(2):145–50.
Li B, Chik CL, Taniguchi N, Ho AK, Karpinski E. 24,25(OH)2 vitamin D3 modulates the L-type Ca2+ channel current in UMR 106 cells: involvement of protein kinase a and protein kinase C. Cell Calcium. 1996;19(3):193–200.
Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17(10):1795–800.
Meszaros JG, Karin NJ, Farach-Carson MC. Voltage-sensitive calcium channels in osteoblasts: mediators of plasma membrane signalling events. Connect Tissue Res. 1996;35(1–4):107–11.
Wang XT, Nagaba S, Nagaba Y, Leung SW, Wang J, Qiu W, et al. Cardiac L-type calcium channel alpha 1-subunit is increased by cyclic adenosine monophosphate: messenger RNA and protein expression in intact bone. J Bone Miner Res. 2000;15(7):1275–85. https://doi.org/10.1359/jbmr.2000.15.7.1275.
Cao C, Ren Y, Barnett AS, Mirando AJ, Rouse D, Mun SH et al. Increased Ca2+ signaling through CaV1.2 promotes bone formation and prevents estrogen deficiency-induced bone loss. JCI Insight. 2017;2(22). doi:https://doi.org/10.1172/jci.insight.95512.
Bergh JJ, Shao Y, Akanbi K, Farach-Carson MC. Rodent osteoblastic cells express voltage-sensitive calcium channels lacking a gamma subunit. Calcif Tissue Int. 2003;73(5):502–10. https://doi.org/10.1007/s00223-002-0016-y.
Meszaros JG, Karin NJ, Akanbi K, Farach-Carson MC. Down-regulation of L-type Ca2+ channel transcript levels by 1,25-dihyroxyvitamin D3. Osteoblastic cells express L-type alpha1C Ca2+ channel isoforms. J Biol Chem. 1996;271(51):32981–5.
Duncan RL, Akanbi KA, Farach-Carson MC. Calcium signals and calcium channels in osteoblastic cells. Semin Nephrol. 1998;18(2):178–90.
Zhang J, Ryder KD, Bethel JA, Ramirez R, Duncan RL. PTH-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated Ca2+ signaling in osteoblasts. J Bone Miner Res. 2006;21(11):1729–37. https://doi.org/10.1359/jbmr.060722.
Duriez J, Flautre B, Blary MC, Hardouin P. Effects of the calcium channel blocker nifedipine on epiphyseal growth plate and bone turnover: a study in rabbit. Calcif Tissue Int. 1993;52(2):120–4.
Jung H, Best M, Akkus O. Microdamage induced calcium efflux from bone matrix activates intracellular calcium signaling in osteoblasts via L-type and T-type voltage-gated calcium channels. Bone. 2015;76:88–96. https://doi.org/10.1016/j.bone.2015.03.014.
Neve A, Corrado A, Cantatore FP. Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol. 2013;228(6):1149–53. https://doi.org/10.1002/jcp.24278.
Wu X, Itoh N, Taniguchi T, Nakanishi T, Tanaka K. Requirement of calcium and phosphate ions in expression of sodium-dependent vitamin C transporter 2 and osteopontin in MC3T3-E1 osteoblastic cells. Biochim Biophys Acta. 2003;1641(1):65–70.
Katz S, Boland R, Santillan G. Purinergic (ATP) signaling stimulates JNK1 but not JNK2 MAPK in osteoblast-like cells: contribution of intracellular Ca2+ release, stress activated and L-voltage-dependent calcium influx, PKC and Src kinases. Arch Biochem Biophys. 2008;477(2):244–52.
Bergh JJ, Xu Y, Farach-Carson MC. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology. 2004;145(1):426–36.
Wen L, Wang Y, Wang H, Kong L, Zhang L, Chen X, et al. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2012;424(3):439–45. https://doi.org/10.1016/j.bbrc.2012.06.128.
Fei D, Zhang Y, Wu J, Zhang H, Liu A, He X et al. Cav 1.2 regulates osteogenesis of bone marrow-derived mesenchymal stem cells via canonical Wnt pathway in age-related osteoporosis. Aging Cell. 2019;18(4):e12967. doi:https://doi.org/10.1111/acel.12967.
Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, et al. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275(50):39193–9. https://doi.org/10.1074/jbc.M006467200.
Ramachandran KV, Hennessey JA, Barnett AS, Yin X, Stadt HA, Foster E, et al. Calcium influx through L-type CaV1.2 Ca2+ channels regulates mandibular development. J Clin Invest. 2013;123(4):1638–46. https://doi.org/10.1172/JCI66903.
Li J, Zhao L, Ferries IK, Jiang L, Desta MZ, Yu X, et al. Skeletal phenotype of mice with a null mutation in Cav 1.3 L-type calcium channel. J Musculoskelet Neuronal Interact. 2010;10(2):180–7.
Cao C, Oswald AB, Fabella BA, Ren Y, Rodriguiz R, Trainor G, et al. The CaV1.2 L-type calcium channel regulates bone homeostasis in the middle and inner ear. Bone. 2019;125:160–8. https://doi.org/10.1016/j.bone.2019.05.024.
Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE(2) production by cultured osteocytes. Am J Physiol-Endocrinol Metab. 1999;276(1):E171–E8.
Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J Biomech. 1996;29(11):1411–7. https://doi.org/10.1016/0021-9290(96)84536-2.
Samnegard E, Cullen DM, Akhter MP, Kimmel DB. No effect of verapamil on the local bone response to in vivo mechanical loading. J Orthop Res. 2001;19(2):328–36. https://doi.org/10.1016/S0736-0266(00)90005-6.
Walker LM, Publicover SJ, Preston MR, Said Ahmed MA, El Haj AJ. Calcium-channel activation and matrix protein upregulation in bone cells in response to mechanical strain. J Cell Biochem. 2000;79(4):648–61.
Walker LM, Holm A, Cooling L, Maxwell L, Oberg A, Sundqvist T, et al. Mechanical manipulation of bone and cartilage cells with ‘optical tweezers’. FEBS Lett. 1999;459(1):39–42.
Thompson WR, Majid AS, Czymmek KJ, Ruff AL, García J, Duncan RL et al. Association of the α2δ1 subunit with Cav3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. Journal of Bone and Mineral Research. 2011;26(9):2125–39. doi:https://doi.org/10.1002/jbmr.437. This was the first work demonstrating a function of auxilliary subunits of VSCCs in bone. This manuscript showed that the α2δ1 subunit associates with T-type (CaV3.2) regulating both trafficking of the pore-subunit to the plasma membrane and mechanically-induced ATP release in osteocytes.
Shao Y, Czymmek KJ, Jones PA, Fomin VP, Akanbi K, Duncan RL, et al. Dynamic interactions between L-type voltage-sensitive calcium channel Cav1.2 subunits and ahnak in osteoblastic cells. Am J Physiol Cell Physiol. 2009;296(5):C1067–C78. https://doi.org/10.1152/ajpcell.00427.2008.
Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280(52):42952–9. https://doi.org/10.1074/jbc.M506415200.
Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20(1):41–9.
Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57(5):344–58. https://doi.org/10.1007/bf00302070.
Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, et al. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42(4):644–52.
Katz S, Boland R, Santillan G. Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in osteoblasts: involvement of mechanical stress-activated calcium influx, PKC and Src activation. Int J Biochem Cell Biol. 2006;38(12):2082–91. https://doi.org/10.1016/j.biocel.2006.05.018.
Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275(13):9645–52. https://doi.org/10.1074/jbc.275.13.9645.
Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28(5):491–8. https://doi.org/10.1016/s8756-3282(01)00415-x.
Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, et al. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276(17):14443–50. https://doi.org/10.1074/jbc.M010021200.
Lou J, Tu Y, Li S, Manske PR. Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem Biophys Res Commun. 2000;268(3):757–62. https://doi.org/10.1006/bbrc.2000.2210.
Mathov I, Plotkin LI, Sgarlata CL, Leoni J, Bellido T. Extracellular signal-regulated kinases and calcium channels are involved in the proliferative effect of bisphosphonates on osteoblastic cells in vitro. J Bone Miner Res. 2001;16(11):2050–6. https://doi.org/10.1359/jbmr.2001.16.11.2050.
Fan X, Rahnert JA, Murphy TC, Nanes MS, Greenfield EM, Rubin J. Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol. 2006;207(2):454–60. https://doi.org/10.1002/jcp.20581.
Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor WR. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002;17(8):1452–60. https://doi.org/10.1359/jbmr.2002.17.8.1452.
Arita NA, Pelaez D, Cheung HS. Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed for the TGFbeta-induced chondrogenic and osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2011;405(4):564–9. https://doi.org/10.1016/j.bbrc.2011.01.068.
Rubin J, Murphy TC, Zhu L, Roy E, Nanes MS, Fan X. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J Biol Chem. 2003;278(36):34018–25. https://doi.org/10.1074/jbc.M302822200.
Thorsen K, Kristoffersson AO, Lerner UH, Lorentzon RP. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. J Clin Invest. 1996;98(11):2446–9. https://doi.org/10.1172/JCI119061.
Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Kim CH, et al. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38(9):1909–17. https://doi.org/10.1016/j.jbiomech.2004.08.009.
Donahue TL, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36(9):1363–71. https://doi.org/10.1016/s0021-9290(03)00118-0.
Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin E(2) by primary bone cells is shear stress dependent. J Biomech. 2001;34(5):671–7. https://doi.org/10.1016/s0021-9290(00)00231-1.
Jee WS, Ma YF. The in vivo anabolic actions of prostaglandins in bone. Bone. 1997;21(4):297–304. https://doi.org/10.1016/s8756-3282(97)00147-6.
Yao W, Jee WS, Zhou H, Lu J, Cui L, Setterberg R, et al. Anabolic effect of prostaglandin E2 on cortical bone of aged male rats comes mainly from modeling-dependent bone gain. Bone. 1999;25(6):697–702. https://doi.org/10.1016/s8756-3282(99)00220-3.
Myers LK, Bhattacharya SD, Herring PA, Xing Z, Goorha S, Smith RA, et al. The isozyme-specific effects of cyclooxygenase-deficiency on bone in mice. Bone. 2006;39(5):1048–52. https://doi.org/10.1016/j.bone.2006.05.015.
Raisz LG. Prostaglandins and bone: physiology and pathophysiology. Osteoarthr Cartil. 1999;7(4):419–21. https://doi.org/10.1053/joca.1998.0230.
Wadhwa S, Choudhary S, Voznesensky M, Epstein M, Raisz L, Pilbeam C. Fluid flow induces COX-2 expression in MC3T3-E1 osteoblasts via a PKA signaling pathway. Biochem Biophys Res Commun. 2002;297(1):46–51. https://doi.org/10.1016/s0006-291x(02)02124-1.
Raisz LG. Physiologic and pathologic roles of prostaglandins and other eicosanoids in bone metabolism. J Nutr. 1995;125(7 Suppl):2024S–7S. https://doi.org/10.1093/jn/125.suppl_7.2024S.
Markovic T, Jakopin Z, Dolenc MS, Mlinaric-Rascan I. Structural features of subtype-selective EP receptor modulators. Drug Discov Today. 2017;22(1):57–71. https://doi.org/10.1016/j.drudis.2016.08.003.
Minamizaki T, Yoshiko Y, Kozai K, Aubin JE, Maeda N. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone. 2009;44(6):1177–85. https://doi.org/10.1016/j.bone.2009.02.010.
Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21(5):294–301. https://doi.org/10.1016/j.tem.2009.12.004.
Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res. 1995;313:36–46.
Weinreb M, Rutledge SJ, Rodan GA. Systemic administration of an anabolic dose of prostaglandin E2 induces early-response genes in rat bones. Bone. 1997;20(4):347–53. https://doi.org/10.1016/s8756-3282(97)00011-2.
Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of different ion channels in osteoblasts ‘and osteocytes’ early responses to mechanical strain. Bone. 1996;19(6):609–14.
Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Phys. 1996;271(1 Pt 1):E205–8. https://doi.org/10.1152/ajpendo.1996.271.1.E205.
Turner CH, Takano Y, Owan I, Murrell GAC. Nitric oxide inhibitor L-NAME suppresses mechanically induced bone formation in rats. Am J Physiol-Endoc M. 1996;270(4):E634–E9.
Helfrich MH, Evans DE, Grabowski PS, Pollock JS, Ohshima H, Ralston SH. Expression of nitric oxide synthase isoforms in bone and bone cell cultures. J Bone Miner Res. 1997;12(7):1108–15. https://doi.org/10.1359/jbmr.1997.12.7.1108.
Wimalawansa SJ. Nitric oxide and bone. Ann N Y Acad Sci. 2010;1192:391–403. https://doi.org/10.1111/j.1749-6632.2009.05230.x.
Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–12. https://doi.org/10.1056/NEJM199312303292706.
Klein-Nulend J, van Oers RF, Bakker AD, Bacabac RG. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos Int. 2014;25(5):1427–37. https://doi.org/10.1007/s00198-013-2590-4.
Kalyanaraman H, Schall N, Pilz RB. Nitric oxide and cyclic GMP functions in bone. Nitric Oxide. 2018;76:62–70. https://doi.org/10.1016/j.niox.2018.03.007.
Fox SW, Chambers TJ, Chow JW. Nitric oxide is an early mediator of the increase in bone formation by mechanical stimulation. Am J Phys. 1996;270(6 Pt 1):E955–60. https://doi.org/10.1152/ajpendo.1996.270.6.E955.
Aguirre J, Buttery L, O'Shaughnessy M, Afzal F, Fernandez de Marticorena I, Hukkanen M et al. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol 2001;158(1):247–257. doi:https://doi.org/10.1016/S0002-9440(10)63963-6.
Bergula AP, Haidekker MA, Huang W, Stevens HY, Frangos JA. Venous ligation-mediated bone adaptation is NOS 3 dependent. Bone. 2004;34(3):562–9. https://doi.org/10.1016/j.bone.2003.11.025.
Armour KE, Armour KJ, Gallagher ME, Godecke A, Helfrich MH, Reid DM, et al. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142(2):760–6. https://doi.org/10.1210/endo.142.2.7977.
Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–63. https://doi.org/10.1124/pr.110.002907.
Rangaswami H, Schwappacher R, Marathe N, Zhuang S, Casteel DE, Haas B et al. Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci Signal. 2010;3(153):ra91. doi:https://doi.org/10.1126/scisignal.2001423.
Rangaswami H, Schwappacher R, Tran T, Chan GC, Zhuang S, Boss GR, et al. Protein kinase G and focal adhesion kinase converge on Src/Akt/beta-catenin signaling module in osteoblast mechanotransduction. J Biol Chem. 2012;287(25):21509–19. https://doi.org/10.1074/jbc.M112.347245.
Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB. Pro-survival effects of 17beta-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem. 2012;287(2):978–88. https://doi.org/10.1074/jbc.M111.294959.
Lin IC, Smartt JM, Jr., Nah HD, Ischiropoulos H, Kirschner RE. Nitric oxide stimulates proliferation and differentiation of fetal calvarial osteoblasts and dural cells. Plast Reconstr Surg 2008;121(5):1554–1566; discussion 67-9. doi:https://doi.org/10.1097/PRS.0b013e31816c3bd7.
Afzal F, Polak J, Buttery L. Endothelial nitric oxide synthase in the control of osteoblastic mineralizing activity and bone integrity. J Pathol. 2004;202(4):503–10. https://doi.org/10.1002/path.1536.
Butler TW. The chemistry and biology of mineralized tissues. Hormone responsiveness of bone cell populations. Birmingham, Alabama: Ebsco Media, Inc.; 1984.
Shao Y, Alicknavitch M, Farach-Carson MC. Expression of voltage sensitive calcium channel (VSCC) L-type Cav1.2 (alpha1C) and T-type Cav3.2 (alpha1H) subunits during mouse bone development. Dev Dyn. 2005;234(1):54–62. https://doi.org/10.1002/dvdy.20517.
Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res. 2012;27(3):563–74. https://doi.org/10.1002/jbmr.1474.
Brown GN, Leong PL, Guo XE. T-Type voltage-sensitive calcium channels mediate mechanically-induced intracellular calcium oscillations in osteocytes by regulating endoplasmic reticulum calcium dynamics. Bone. 2016;88:56–63. https://doi.org/10.1016/j.bone.2016.04.018.
Verkhratsky A. Calcium and cell death. Subcell Biochem. 2007;45:465–80.
Liu L, Li H, Cui Y, Li R, Meng F, Ye Z, et al. Calcium channel opening rather than the release of ATP causes the apoptosis of osteoblasts induced by overloaded mechanical stimulation. Cell Physiol Biochem. 2017;42(2):441–54. https://doi.org/10.1159/000477592.
Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98–108. doi:21/1/98 [pii].
Tanaka T, Nangaku M, Miyata T, Inagi R, Ohse T, Ingelfinger JR, et al. Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol. 2004;15(9):2320–33. https://doi.org/10.1097/01.ASN.0000138287.46849.82.
Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(3):563–74. https://doi.org/10.1002/jbmr.1474.
Lewis KJ, Frikha-Benayed D, Louie J, Stephen S, Spray DC, Thi MM et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proc Natl Acad Sci U S A. 2017;114(44):11775–80. doi:https://doi.org/10.1073/pnas.1707863114. Lewis and colleagues used intravital imaging to delineate the responses of Ca2+ influx in osteocytes in response to in vivo mechanical loading. Modulation of the frequency and magnitude of loading in real-time provided critical insight into the function of Ca2+ signaling in bone.
Jing D, Baik AD, Lu XL, Zhou B, Lai X, Wang L, et al. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014;28(4):1582–92. https://doi.org/10.1096/fj.13-237578.
Ishihara Y, Sugawara Y, Kamioka H, Kawanabe N, Kurosaka H, Naruse K, et al. In situ imaging of the autonomous intracellular Ca(2+) oscillations of osteoblasts and osteocytes in bone. Bone. 2012;50(4):842–52. https://doi.org/10.1016/j.bone.2012.01.021.
Kajiya H, Okamoto F, Fukushima H, Takada K, Okabe K. Mechanism and role of high-potassium-induced reduction of intracellular Ca2+ concentration in rat osteoclasts. Am J Physiol Cell Physiol. 2003;285(2):C457–66.
Fan P, Hu N, Feng X, Sun Y, Pu D, Lv X et al. Cav1.3 is upregulated in osteoporosis rat model and promotes osteoclast differentiation from preosteoclast cell line RAW264.7. J Cell Physiol. 2019;234(8):12821–7. doi:https://doi.org/10.1002/jcp.27937. This work demonstrated that osteoporosis accompanies increased expression of L-type VSCCs. Knockdown of CaV1.3 inhibits osteoclast differentiation and activity.
Suda T, Tanaka S, Takahashi N. Macrophage colon-stimulating factor (M-CSF) is essential for differentiation rather than proliferation of osteoclast progenitors. Osteoporos Int. 1993;3(Suppl 1):111–3. https://doi.org/10.1007/bf01621881.
Takayanagi H, Kim S, Taniguchi T. Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res. 2002;4(Suppl 3):S227–32. https://doi.org/10.1186/ar581.
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. https://doi.org/10.1016/s1534-5807(02)00369-6.
Mentaverri R, Yano S, Chattopadhyay N, Petit L, Kifor O, Kamel S, et al. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J. 2006;20(14):2562–4. https://doi.org/10.1096/fj.06-6304fje.
Boudot C, Saidak Z, Boulanouar AK, Petit L, Gouilleux F, Massy Z, et al. Implication of the calcium sensing receptor and the Phosphoinositide 3-kinase/Akt pathway in the extracellular calcium-mediated migration of RAW 264.7 osteoclast precursor cells. Bone. 2010;46(5):1416–23. https://doi.org/10.1016/j.bone.2010.01.383.
Kaji H, Sugimoto T, Kanatani M, Chihara K. High extracellular calcium stimulates osteoclast-like cell formation and bone-resorbing activity in the presence of osteoblastic cells. J Bone Miner Res. 1996;11(7):912–20. https://doi.org/10.1002/jbmr.5650110707.
Ng PY, Brigitte Patricia Ribet A, Pavlos NJ. Membrane trafficking in osteoclasts and implications for osteoporosis. Biochem Soc Trans 2019;47(2):639–650. doi:https://doi.org/10.1042/BST20180445.
Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJ, Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun. 2004;315(4):823–9. https://doi.org/10.1016/j.bbrc.2004.01.138.
Fan X, Roy E, Zhu L, Murphy TC, Ackert-Bicknell C, Hart CM, et al. Nitric oxide regulates receptor activator of nuclear factor-kappaB ligand and osteoprotegerin expression in bone marrow stromal cells. Endocrinology. 2004;145(2):751–9. https://doi.org/10.1210/en.2003-0726.
Turner CH, Owan I, Jacob DS, McClintock R, Peacock M. Effects of nitric oxide synthase inhibitors on bone formation in rats. Bone. 1997;21(6):487–90. https://doi.org/10.1016/s8756-3282(97)00202-0.
Funding
1R01AR074473-01 to WRT, MCFC, and AGR; R15AR069943-01 to WRT, and 1F32AR074893-01 to CSW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skeletal Biology and Regulation
Rights and permissions
About this article
Cite this article
Wright, C.S., Robling, A.G., Farach-Carson, M.C. et al. Skeletal Functions of Voltage Sensitive Calcium Channels. Curr Osteoporos Rep 19, 206–221 (2021). https://doi.org/10.1007/s11914-020-00647-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00647-7